Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 5
Pegylated liposomal doxorubicin for PIK3CA “wild”, HR+, HER2-, chemoresistant advanced breast cancer
Movchan Oleksii Volodimirovich*, Smolanka Ivan Ivanovich, Lyashenko Andriy Oleksandrovich, Loboda Anton Dmitrovich, Dosenko Irina Viktorivna and Ivankova Oksana MykolaivnaMovchan Oleksii Volodimirovich, Department of Breast cancer and Reconstructive surgery, State Non-commercial Enterprise “National Cancer Institute” of the Ministry of Health of Ukrai, Ukraine, Email: aleexeymed@gmail.com
Received: 08-Feb-2025, Manuscript No. OAR-25-161061 ; , Pre QC No. P-161061 ; Editor assigned: 10-Feb-2025, Pre QC No. P-161061 ; Reviewed: 24-Feb-2025, QC No. Q-161061 ; Revised: 03-Apr-2025, Manuscript No. R-161061 ; Published: 30-Apr-2025
Abstract
Introduction: In 2023, nearly 19 million individuals were newly diagnosed with cancer, with breast cancer accounting for 12.7% of all cases. Many efforts were made to reduce the negative effects of doxorubicin while increasing its efficacy. Pegylated Liposomal Doxorubicin (PLD) is a medicine that claims to fulfill these two objectives due of its distinct pharmacokinetic characteristics. Differential activity in patients with PIK3CA-altered vs. PIK3CA-wild-type, estrogen receptor-positive metastatic breast cancer that has progressed during or after antiestrogen treatments. By conducting a comparison analysis with typical anthracycline regimens, this study, using data from earlier studies, tries to understand the possible benefits of PLD.
Purpose: To evaluate the efficacy and safety of Pegylated Liposomal Doxorubicin (PLD) in patients with PIK3CA-wild, HR+, HER-, Chemo resistant Advanced Breast Cancer (CABC), treated with anthracycline and taxanes.
Material and methods: First group (wild PIK3CA) – 30 patients were treated with pegylated liposomal doxorubicin 50 mg/m2 diluted in 5% dextrose 250 ml intravenous infusion for 1 hour on day every 28 days – four cycles; second group (mutant PIK3CA) – 30 patients were treated with pegylated liposomal doxorubicin 50 mg/m2 diluted in 5% dextrose 250 ml intravenous infusion for 1 hour on day every 28 days – four cycles.
Results: The PIK3CA-mutant arm had a median OS of 32.54 months (HR=0.76; 95% CI, 0.68-0.86; one-sided P=0.08) compared to 31.34 months in PIK3CAwild arm (HR=0.78; 95% CI, 0.62-0.98; one-sided P=0.06).
Conclusions: By evaluating all patients treated with single-agent PLD at our Research Institute, we demonstrated that the outcomes of PLD in patients with CABC HR+HER2-PIK3CAwild did not match previously published data from prospective observational studies. The observed results in PIK3CA-wild were not significantly inferior to the total population. PLD remains a viable therapy option in these more sensitive patient populations. PLD-based neoadjuvant chemotherapy may give considerable benefits in terms of OS for CABC patients. The findings highlight the possibility of this treatment regimen as a more effective and probably less toxic alternative to traditional chemotherapy, with long-term advantages that might change existing treatment procedures.
Keywords
Pegylated liposomal doxorubicin; PIK3CA mutations; PIK3CA-Wild; Hormone receptor-positive аdvanced breast cancer; Efficacy comparisons; Challenges and treatment options
INTRODUCTION
Breast cancer is one of the most common cancers affecting women globally, with serious consequences for patient survival and quality of life. In 2023, nearly 19 million individuals were newly diagnosed with cancer, with breast cancer accounting for 12.7% of all cases [1]. Neoadjuvant chemotherapy has become a critical treatment for advanced breast cancer. It is meant to reduce tumor size preoperatively, allowing breast-conserving surgery and improving surgical results [2]. Anthracyclines, such as doxorubicin, are widely regarded as essential in the treatment of breast cancer at all stages. However, the risk of cumulative dose-dependent irreversible cardiotoxicity and other dose-limiting toxicities, such as nausea and vomiting, outweighs their usefulness [3]. Many efforts were made to reduce the negative effects of doxorubicin while increasing its efficacy. Pegylated Liposomal Doxorubicin (PLD) is a medicine that claims to fulfill these two objectives due of its distinct pharmacokinetic characteristics [4]. Although PLD has a lower toxicity profile than traditional anthracyclines, it is more appropriate alternative for individuals with Chemoresistant Advanced Breast Cancer (CABC), especially those with risk factors for cardiac illness [5].
According to the National Comprehensive Cancer Network's clinical recommendations for breast cancer, the recommended dose of PLD is 50 mg/m2 day 1 every 4 weeks [6]. PLD's unique pharmacokinetic features and liposomal encapsulation enable prolonged circulation duration and selective tumor absorption. This is thought to increase antitumor effectiveness while reducing exposure to healthy tissue [7]. Recent clinical studies have indicated that PLD followed by docetaxel as neoadjuvant treatment for advanced breast cancer resulted in a pCR rate of 17.9% with no significant change in the left ventricular ejection fraction [8]. Similarly, PLD followed by paclitaxel yielded a pCR rate of 33% in patients prone to cardiotoxicity with high-risk breast cancer, increasing the likelihood of conservative surgery from 24 to 59% [9].
Mutations in the PIK3CA gene are found in around 40% from Advanced Breast Cancer (ABC) that is Hormone Receptor positive (HR+), but human epidermal growth factor receptor 2 negative. PIK3CA mutations cause hyperactivation of the Phosphoinositide 3-Kinase (PI3K) signaling pathway, treatment resistance, and poor prognosis [10]. Differential activity in patients with PIK3CA-altered vs. PIK3CA-wild-type, estrogen receptor-positive metastatic breast cancer that has progressed during or after antiestrogen treatments [11]. In PIK3CA-mutated ABC patients, alpelisib with fulvestrant plays a vital role; nevertheless, the question for individuals without PIK3CA-mutated illness remains unanswered [12].
Given the burden of BC and the need to find treatments that balance efficacy and safety, the purpose of this study is to assess the impact of PLD-based neoadjuvant chemotherapy on Overall Survival (OS) and Disease-Free Survival (DFS) among chemoresistant advanced breast cancer patients based on PIK3CA status [13]. By conducting a comparison analysis with typical anthracycline regimens, this study, using data from earlier studies, tries to understand the possible benefits of PLD [14].
Purpose: To evaluate the efficacy and safety of Pegylated Liposomal Doxorubicin (PLD) in patients with PIK3CA-wild, HR+, HER-, Chemoresistant Advanced Breast Cancer (CABC), treated with anthracycline and taxanes.
MATERIALS AND METHODS
We identified patients with Chemoresistant Advanced Breast Cancer (CABC) having received single-agent PLD at the State non-commercial entertainment “National Cancer Institute of Ukraine” between January 01, 2023 and January 01, 2026. Electronic patient charts were viewed, and clinical data points were collected in an anonymized database. Last update was on January 31, 2026. This study was approved by the responsible ethics committee, State non-commercial entertainment “National Cancer Institute of Ukraine” December 12, 2023, № 249/2. This study was conducted according to the Declaration of Helsinki and Good Clinical Practice Guidelines. Patient consent regarding use of health-related data for research purposes was available from all patients.
Inclusion criteria for this study were: women, whose disease progressed, or stabilizated (with histopathological confirmation and radiologically confirmed chemoresistant advanced disease) after chemotherapy for postmenopausal advanced luminal BC with two lines (AC–T) and concomitant Letrozole without distant metastases. Patients received at least two doses of Pegylated Liposomal Doxorubicin (PLD) for САBC at our clinic.
Patients randomized 1:1 to receive as sequential neoadjuvant therapy: First group (wild PIK3CA) – 30 patients were treated with pegylated liposomal doxorubicin 50 mg/m2 diluted in 5% dextrose 250 ml intravenous infusion for 1 hour on day every 28 days – four cycles;
Second group (mutant PIK3CA) – 30 patients were treated with pegylated liposomal doxorubicin 50 mg/m2 diluted in 5% dextrose 250 ml intravenous infusion for 1 hour on day every 28 days – four cycles.
Treatment was continually administered until disease progression, unacceptable toxicity, treatment delay of > 3 weeks owing to toxicity, completion of 4 cycles, or patient’s decision to withdraw from the study. These chemotherapy protocols were established following the National Comprehensive Cancer Network guidelines for breast cancer [6]. Post-chemotherapy, targeted patients also received radiation and endocrine therapy.
Patient assessment
Clinical information recorded for this study encompassed the date of diagnosis, age at diagnosis, dates of recurrence and death, clinical stage, molecular subtype, PIK3CA gene mutation, tumor size, and menopause status. Response data were taken from routine CT-scans and was defined according to RECIST 1.1 criteria. Tumor assessments were conducted using mammography, ultrasound, CT, and breast MRI. The clinical stage of the tumor was determined according to the TNM, 8th Edition. Toxicity was graded following the American Society of Clinical Oncology (ASCO) standards [15]. Patients undergoing PLD treatment were monitored after each treatment cycle. Cardiac function, specifically Left Ventricular Ejection Fraction (LVEF), was measured using echocardiography, and Electrocardiogram (ECG) readings were also obtained during the study.
Statistics
Overall Survival (OS) was defined as the interval from the initiation of treatment to the date of death. Patients who were alive or without disease progression at study closure were censored as of the last follow-up date. Considering these parameters, a sample size of the least 45 patients was necessary. Therefore, we aimed to recruit 60 patients. Patients who received at least two doses of PLD and completed at least one efficacy evaluation were included in the efficacy analysis. OS analyses were estimated using the Kaplan–Meier method, and 95% Confidence Intervals (CIs) were also calculated. Descriptive quantitative data were expressed as median and range according to the data distribution, and qualitative data were expressed as counts and percentages. Two-sided p values were reported and p values less than 0.05 were considered significant. Predefined variables were age, relevant comorbidity (congestive heart failure, coronary heart disease, hypertension, chronic pulmonary disease, chronic kidney failure, diabetes), HR-status, Her2-status, tumor grade. All statistical analyses were performed with SPSS version 28 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Discontinuation reason was progressive disease: 2 (6.67%) in the PIK3CA-mutant and 1 (3.33%) of patients in the PIK3CA-wild arms, respectively. Discontinued the treatment phase due to an Adverse Event (AE): 6.67% (n=2) for the PIK3CA-mutant group and 10.00% (n=3) for the PIK3CA-wild group.
The median follow-up time for OS (from randomization to incident or censoring) was 36.00 months. The PIK3CA-mutant arm had the three-years OS PIK3CA-mutant 83.4% (HR=0.76; 95% CI, 0.68-0.86; one-sided P=0.08) compared to 92.9% in PIK3CA-wild arm (HR=0.78; 95% CI, 0.62-0.98; one-sided P=0.06) (Fig. 1.).
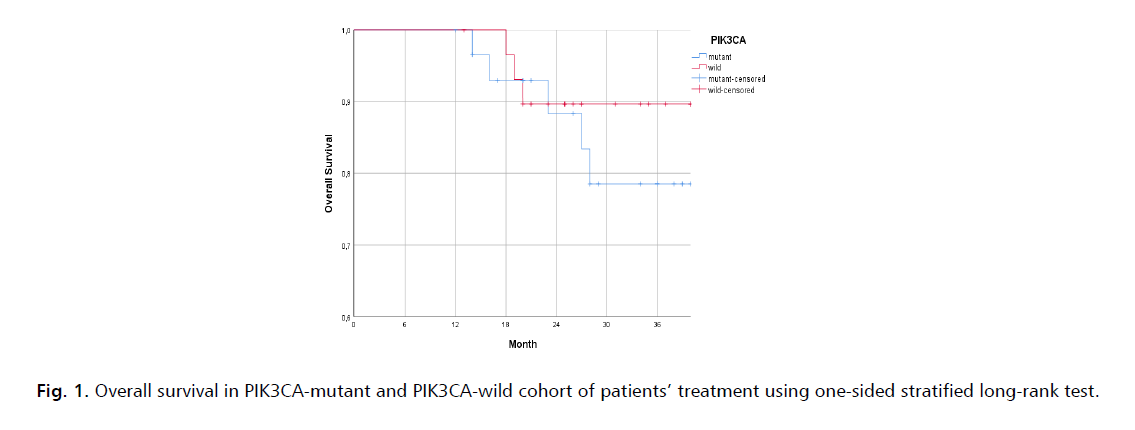
Figure 1: Overall survival in PIK3CA-mutant and PIK3CA-wild cohort of patients’ treatment using one-sided stratified long-rank test.
Research stratification criteria for lymph node metastases conducted for OS analyze. In patients with lymph node metastases, the 3-years OS PIK3CA-mutant 78,5% was 30.03 months (95% CI, 24.26-36.00) in the PIK3CA-mutant group and 89,7% (95% CI, 20.72-36.00) in the PIK3CA-wild group (Fig. 2.).
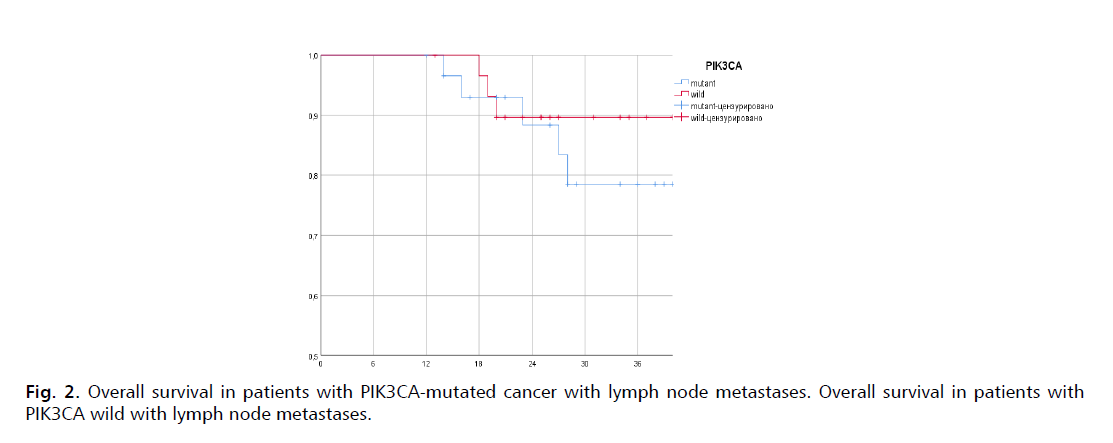
Figure 2: Overall survival in patients with PIK3CA-mutated cancer with lymph node metastases. Overall survival in patients with PIK3CA wild with lymph node metastases.
From sixty patients with ER+, HER2– breast cancer and measurable target lesions per Response Evaluation Criteria in Solid Tumors, version 1.0: two patients (3.33%) had comlete response 47 (78.33%) achieved partial response, and 3 (5%) patients had process stabilization.
Of 30 patients with measurable disease and samples containing somatic genetic alterations of known or likely significance on central assessment (PIK3CA-mut), 22 (73.33%) achieved partial response, and stabilization had 3 (13.33%), and none comlete response.
From thirty patients with PIK3CA mutation absence (PIK3CA-wild), two patients (6.67%) had complete response, therefore 25 (83.33%) achieved partial response, and stabilization had 1 (3.33%) patient, p=0.06 (Fig. 3.).
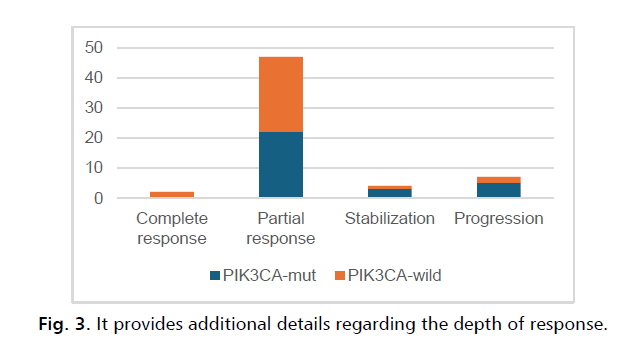
Figure 3: It provides additional details regarding the depth of response.
This research had a favorable overall safety profile. There were no grade 4/5 adverse events recorded. The most often reported adverse events were leukopenia, tiredness, and neutropenia. The most prevalent grade 3 Adverse Events (AEs) were neutropenia (1 patient, 3.33%), tiredness (1 patient, 3.33%), and PIK3CA-wild (2 patients, 6.67%). No patients saw a substantial decline in LVEF during or after the four PLD sessions.
DISCUSSION
We aimed to characterize our patient population, assess outcomes, and conduct group analyses in patients who had previously received neoadjuvant chemotherapy to mitigate the unwanted biological effects of PLDs by prolonging blood circulation and deposition per tumor. This trial looked at the effectiveness and safety of PLD monotherapy at 50 mg/m2 every 4 weeks in women with HER2-negative BC, depending on PIK3CA status, who had been substantially pretreated with conventional anthracycline and taxanes. Guarneri V and colleagues denotes, that there is an unmet need for patients with severely pretreated HER2-negative ABCs. After anthracycline and taxane failure, no optimum chemotherapy treatment has been found [16]. Single-agent PLD exhibited efficacy and safety for the treatment of chemoresistant ABC [17].
SOLAR-1 investigated OS as a secondary objective in patients with HR+, HER2−, and PIK3CA-mutated hormoneresistant ABC. Adding alpelisib to fulvestrant improved OS by 7.9 months, although the difference was not statistically significant. The addition of alpelisib to fulvestrant extended the median time to first treatment by 8.5 months. With a longer follow-up period, the alpelisib with fulvestrant safety profile remained consistent, with no new safety signals [18], but we had still no data about HR+Her2-PIK3CAwild ABC, therefore results from our study are inspired: thirty patients with PIK3CA-wild, two patients (6.67%) had complete response, therefore 25 (83.33%) achieved partial response, p=0,06.
For patients with pretreated BC (Then chemoresistance developed), PLD 50 mg/m2 every 4 weeks as a third-line treatment by Al-Batran, et al. [18] recorded median OS of 11.2 months (95% CI 6.4 –17.0). Another multicenter phase II study of patients with CABC all previously treated with conventional anthracyclines and 71.4% with prior taxane exposure who received PLD 50 mg/m2 every 4 weeks showed OS 13.5 months (95% CI 8.8–19.2) [19]. In our analysis, the OS was 32.54 months (HR=0.76; 95% CI, 0.68-0.86; one-sided P=0.08), which is quantitatively longer than the trials listed above. Notably, all patients in our trial had previously taken standard anthracycline and taxane.
The results of the current trial revealed that anthracycline- and taxane-resistant BC is not cross-resistant to PLD, comparable to the findings of a randomized phase III investigation of patients with taxane-refractory advanced breast cancer [20]. There was no significant decline in LVEF throughout therapy after four cycles of PLD. These results were consistent with earlier investigations using PLD 10 mg/m2 per week [21]. As a result, a sampling bias that would disfavor the real patient population observed in daily practice. To examine this subject, we sought to give a full treatment experience by examining all CABC patients treated with PLD.
LIMITATIONS
To begin, this trial was conducted in a single center using an open-label design with no control arm. Second, this was an exploratory experiment with a limited sample size.
CONCLUSION
By evaluating all patients treated with single-agent PLD at our Research Institute, we demonstrated that the outcomes of PLD in patients with CABC HR+HER2-PIK3CAwild did not match previously published data from prospective observational studies. The observed results in PIK3CA-wild were not significantly inferior to the total population. PLD remains a viable therapy option in these more sensitive patient populations. PLD-based neoadjuvant chemotherapy may give considerable benefits in terms of OS for CABC patients. The findings highlight the possibility of this treatment regimen as a more effective and probably less toxic alternative to traditional chemotherapy, with long-term advantages that might change existing treatment procedures.
AUTHOR CONTRIBUTIONS
Movchan O.V.(MOV), Prof. Smolanka I.I.(SII), Lyashenko A.O. (LAO), Dosenko I.V.(DIV), Loboda A.D (LAD), Ivankova O.M.(IOM).
MOV: Conceptualization, project administration, writing-original draft, writing—review and editing; MOV, LAO: Investigation, writing—review and editing; SII, LAO: Data curation: DIV, LAD, IOM: Validation, MOV, LAO: Formal analysis, MOV, LAD: Software. All authors have read and agreed to the published version of the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- World Health Organization. Global cancer burden growing, amidst mounting need for services. World Health Organization: Geneva, Switzerland. 2024.
- Baskin AS, Huppert LA, Kelil T, Singer L, Mukhtar RA. The Neoadjuvant Approach to Treatment of Breast Cancer: Multidisciplinary Management to Improve Outcomes. Surg Oncol Insight. 2024; 18:100059.
- Sandamali JA, Hewawasam RP, Fernando MA, Jayatilaka KA. Electrocardiographic and biochemical analysis of anthracycline induced cardiotoxicity in breast cancer patients from Southern Sri Lanka. BMC cancer. 2023; 23(1):210.
- Taher M, Susanti D, Haris MS, Rushdan AA, Widodo RT, et al. PEGylated liposomes enhance the effect of cytotoxic drug: A review. Heliyon. 2023; 9(3).
- Volodimirovich MO, Ivanovich SI, Oleksandrovich LA, Viktorivna DI, Dmitrovich LA, et al. PIK3CA mutation presence as luminal breast cancer chemoresistance prognostic marker. Onkologia i Radioterapia. 2024; 18(2).
- of National BC, for Cancer QC, of China BC, Anti-Cancer Association, Cancer Drug Clinical Research Committee of China Anti-Cancer Association. Guidelines for diagnosis and treatment of advanced breast cancer in China (2022 edition). J Natl Cancer Cent. 2024; 4(2):107-127.
- Fan D, Cao Y, Cao M, Wang Y, Cao Y, et al. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023; 8(1):293.
- Yang Y, Jin L, Li Y, Rao N, Gong C, et al. Sequential neoadjuvant chemotherapy using pegylated liposomal doxorubicin and cyclophosphamide followed by taxanes with complete trastuzumab and pertuzumab treatment for HER2-positive breast cancer: A phase II single-arm study. Chin J Cancer Res. 2024; 36(1):55.
- Lu H, Yan H, Liao S, Deng J, Zhang J, et al. Efficacy, cardiotoxicity and factors affecting pathologic complete response of neoadjuvant chemotherapy with anthracycline-containing verses anthracycline-free regimens plus dual HER2 blockade for HER2-positive early-stage breast cancer: A retrospective study. Transl Cancer Res. 2023; 12(6):1490.
- Chen JW, Murugesan K, Newberg JY, Sokol ES, Savage HM, et al. Comparison of PIK3CA mutation prevalence in breast cancer across predicted ancestry populations. JCO Precision Oncol. 2022; 6:e2200341.
- Alves CL, Ditzel HJ. Drugging the PI3K/AKT/mTOR pathway in ER+ breast cancer. Int J Mol Sci. 2023; 24(5):4522.
- André F, Ciruelos EM, Juric D, Loibl S, Campone M, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021; 32(2):208-217.
- Chun SJ, Jang BS, Choi HS, Chang JH, Shin KH, et al. Prediction of Overall Disease Burden in (y) pN1 Breast Cancer Using Knowledge-Based Machine Learning Model. Cancers. 2024; 16(8):1494.
- Hurvitz SA, McAndrew NP, Bardia A, Press MF, Pegram M, et al. A careful reassessment of anthracycline use in curable breast cancer. NPJ Breast Cancer. 2021; 7(1):134.
- ASCO. Guidelines, Tools, & Resources. Breast cancer. Reviewed 2024.
- Guarneri V, de Azambuja E. Anthracyclines in the treatment of patients with early breast cancer. ESMO open. 2022; 7(3):100461.
- Health Commission of the People's Republic Of China N. National guidelines for diagnosis and treatment of breast cancer 2022 in China (English version). Chin J Can Res= Chung-kuo yen Cheng yen Chiu. 2022; 34(3):151-175.
- Al-Batran SE, Bischoff J, Von Minckwitz G, Atmaca A, Kleeberg U, et al. The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: A multicentre phase II trial. British J Can. 2006; 94(11):1615-20.
- Fiegl M, Mlineritsch B, Hubalek M, Bartsch R, Pluschnig U, et al. Single-agent Pegylated Liposomal Doxorubicin (PLD) in the treatment of metastatic breast cancer: Results of an Austrian observational trial. BMC cancer. 2011; 11:1-9.
- Keller AM, Mennel RG, Georgoulias VA, Nabholtz JM, Erazo A, et al. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol. 2004; 22(19):3893-3901.
- Ji Y, Zhang X, Liu J, Chen Y, Meng M, et al. Direct quantitation of free, encapsulated, total doxorubicin and doxorubicinol in stabilized frozen human plasma to support a BE study of liposomal doxorubicin. J Pharm Biomed Anal. 2020; 189:113388.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at



