Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 6
Preparation and characterization of Eudragit L30 D-55, L100 and S100 coated Montelukast sodium pellets for chronotherapy: A possible relevance to therapeutic targets
Ponnaganti SS Prasanna Kumar and Lankalapalli Srinivas*Lankalapalli Srinivas, Department of Pharmaceutics, GITAM School of Pharmacy, GITAM Deemed to be University, Rushikonda, Visakhapatnam, India, Email: slankala@gitam.edu
Received: 02-Jan-2025, Manuscript No. OAR-25-160820 (R); , Pre QC No. OAR-25-160820 (PQ); Editor assigned: 07-Jan-2025, Pre QC No. OAR-25-160820 (PQ); Reviewed: 21-Jan-2025, QC No. OAR-25-160820; Revised: 05-Aug-2025, Manuscript No. OAR-25-160820; Published: 02-Sep-2025
Abstract
Objective: The initial research investigation on the current research work was to design, develop oral formulation of Pulsatile Drug Delivery Systems (PDDS) for Montelukast sodium with aim of treatment for asthma and designed as Pellets type of system with immediate and extended release of drug occurs after the lag time period. In chronic asthma condition patients suffered during the early morning hours (04.00 am to 06.00 am) so immediate release dosage forms are not suitable but the modified release formulation that is given at bed time and releases the drug as pulsed manner despite to wake up the patient.
Methods: In this present investigation, Montelukast sodium pellets were prepared using Fluidized Bed Processor (FBP), seal coating with HPMC E5 and enteric coatings with Eudragit L30 D-55, Eudragit L100 and Eudragit S100 polymers for extending lag period prior the drug release.
Results: The drug-excipient compatibility for the formulation was assessed using FTIR and DSC; the results obtained shown that no chemical interactions. The optimised formulations of IR Pellets MEF-6 showed release of drug 99% at 6 h with 4.5 h lag time and ER Pellets MXR-8 showed 99% of drug release at 12 h with 05 h lag time. SEM Studies showed uniform surface morphology on the prepared coated pellets. As per ICH standards for optimized formulations, stability experiments were also conducted for 6 months at 40ºC at 75% RH and it was concluded to be stable.
Conclusion: The designed pellets of both IR and ER release with enteric coatings beneficial to patients with night time dosing that can prevent the asthma episodes at early morning and development of exacerbations serving as effective therapy for various diseases.
Keywords
Pulsatile; Lag time; Fluid bed processor; Enteric coating; Circadian rhythms
Introduction
Oral drugs are the largest delivery segment of the overall drug delivery market. A Pulsatile release profile is one which the drug release rapidly at once following a predetermined lag time; this is beneficial when implementing many therapies to address therapeutic demands related to circadian rhythms of disease or body functions [1,2].
Pulsatile drug delivery profile is one of the therapeutic target used in anticancer therapies. Hyper responsiveness to a variety of stimuli is characteristic for asthma which is a long-term inflammatory disorder of the airways. Chronotherapy of asthma aims to maximize the benefits of bronchodilator drugs in early morning hours due to the importance of circadian rhythms. The pulsatile systems were designed as time release pattern of drug and it is helpful for the preventing asthmatic attacks at early morning and to enhance the patient compliance nature and dosing frequency is reduced [3,4].
Now a days multi particulate dosage forms due to their potential advantages including gastric emptying, convenient release patterns, reduction in chance of dose dumping, and enhanced bioavailability with minimum inter, intra subject variability are having great importance versus single-unit dosage forms. Pellets have been described as spherical, granules of free-flowing size varying from 500 and 1500 μm showed reduced friable dosage form, ease of coating and packed uniformly [5,6].
T his familiarity is because of fast dispersion of formulated pellets in the GI tract with absorption is maximum, reduction of fluctuations in plasma peak and minimize side effects by maintaining drug bioavailability [7,8].
T he mast cells and eosinophils releases eicosanoids such as Cysteinyl Leukotrienes (CysLT) like LT C4, D4 and E4. The released CysLT bind to CysLT type-1 receptors present on smooth muscle cells, macrophages in respiratory airway and eosinophils of pro inflammatory cells and some specific myeloid stem cells activities. T his binding facilitates the stimulation in conditions like asthma and allergic rhinitis. In particular, asthma is facilitated by types of effects such as CysLT-mediated airway bronchoconstriction, mucous secretion along with vascular permeability and eosinophil recruitment [9-11].
Materials and Methods
Materials
Montelukast Sodium (MKS) has been supplied by the Seldom Pharma PVT Ltd (Yanam, Pondicherry, India) as a gift sample. HPMC E5, Sodium starch Glycolate, Eudragit L 30 D 55, L 100 and S100, Eudragit RSPO, Talc, Polysorbate 80, Triethyl citrate were obtained from Therallen Pharma PVT Ltd, Hyderabad as a gift sample respectively. The chemicals incorporated along with API were analytical grade.
Methods
Preparation of Immediate Release (IR) pellets with drug loading, seal coating and enteric coatings
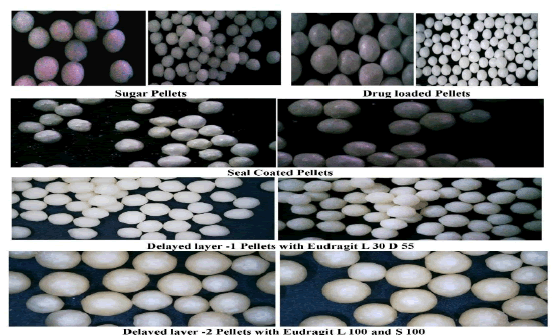
Drug loading: The formulation of Montelukast pellets were initiated with drug loading by Fluidized Bed Process (FBP). Five different formulations (MDL-1 to MDL-5) for drug loading on sugar spheres of size 20/25. All formulations were formulated by using equipment fluid bed coater. So, the sugar spheres of core were loaded in the fluidized bed coater and drug loading can be done by MKS, SSG, HPMC E5, Talc, PEG 400 dissolved in purified water to make coating solution and coated on the sugar spheres. The pellets were filled in capsules. The FBP machine parameters illustrated in Table 1 for drug loading, seal coating and enteric coatings maintained in such a way to avoid twinning of pellets and smooth surface around pellets and formulations of drug loading illustrated in Table 2.
| S. no | Parameter | Drug loading | Seal coating | Enteric coatings |
|---|---|---|---|---|
| 1 | Inlet temperature (°C) | 50 | 50 | 50 |
| 2 | Bed temperature (°C) | 45 | 45 | 40 |
| 3 | Exhaust temperature (°C) | 43 | 43 | 42 |
| 4 | Atomization (Bar) | 1.2 | 1 | 1 |
| 5 | Spray rate (g/min) | 5-7 | 5-6 | 5-7 |
| 6 | Air flow (cfm) | 1500-2500 | 1500-2500 | 2000-2500 |
Tab. 1. Fluid bed coater processing parameters in coatings
|
|
|
mg/Capsule |
|||||
|---|---|---|---|---|---|---|---|
|
S. no |
Ingredients |
Functional category |
MDL-1 |
MDL-2 |
MDL-3 |
MDL-4 |
MDL-5 |
|
1 |
Sugar spheres (20#/25#) |
|
100 |
100 |
100 |
100 |
100 |
|
Drug loading |
|||||||
|
2 |
Montelukast Sodium |
API |
10 |
10 |
10 |
10 |
10 |
|
3 |
SSG |
Disintegrant |
4 |
4 |
6 |
6 |
8 |
|
4 |
HPMC E5 |
Binder |
4 |
6 |
8 |
10 |
10 |
|
5 |
Talc |
Smoothing |
1 |
1 |
1 |
1 |
1 |
|
6 |
PEG 400 |
Plasticizer |
1 |
1 |
1 |
1 |
1 |
|
7 |
Purified water (ml) |
Vehicle |
Q. S |
Q. S |
Q. S |
Q. S |
Q. S |
|
Drug pellets weight |
120 |
122 |
126 |
128 |
130 |
||
Tab. 2. Formulations of IR drug loading pellets.
Seal coating: From the above formulations of drug loading, the optimized formulation selected based on the results of drug loading efficiency, shape of pellets and in vitro dissolution studies seal coating was done by using HPMC E 5 same FBP process on optimized formulation and formulations illustrated in Table 3.
| S. no | Ingredients | Functional category | MDS-1 (mg/capsule) |
|---|---|---|---|
| 1 | Selected drug loading pellets | 130 | |
| Seal coating | |||
| 2 | HPMC E5 | Binder | 9 |
| 3 | Talc | Smoothing | 1 |
| 4 | PEG 400 | Plasticizer | 1 |
| 5 | Purified water | Vehicle | QS |
| Drug pellets weight in mg | 141 | ||
Tab. 3. Seal coating on selected drug loaded pellets.
Enteric coatings: I and II: The seal coated pellets form MDS-1 were taken for the enteric coating for delaying the drug release. The selection of polymers was mainly based on the intestinal pH and its transit time. For enteric coating three different polymers were selected as Eudragit L30 D 55, L 100 and S 100. These three polymers are having pH dependent solubility. The duodenum, jejunum and ileum pH are important for the chrono modulated delivery system development. The enteric coating on the seal coated pellets with help of FBP in 02 layers. Layer-I was given with Eudragit L 30 D 55 (MEA-1 to MEA-3) and Layer- II with Eudragit L 100, Eudragit S 100 (MEF-1 to MEF-6). The weight of the pellets was adjusted for enteric coatings based on the drug content values of the enteric coating layer-I pellet for Enteric coating II layer. The prepared pellets were dried at 35°C upto 5-6 hrs for the complete evaporation of the moisture content. The pellets were filled into capsule shells, the weight of the pellets was adjusted based on the assay values for dissolution process. The formulations of Enteric coatings were illustrated in Tables 4 and 5.
| Enteric layer-I coating | mg/capsule | ||||
|---|---|---|---|---|---|
| S. no | Ingredients | Functional category | MEA-1 | MEA-2 | MEA-3 |
| 1 | Seal coated pellets (MDS-1) |
141 | 141 | 141 | |
| 2 | Eudragit L 30 D 55 | Delaying drug release | 32.99 | 49.35 | 62 |
| 3 | Triethyl citrate | Solubilizer, emulsifier | 3.00 | 4.50 | 6.00 |
| 4 | Polysorbate 80 | Plasticizer | 0.55 | 0.85 | 1.15 |
| 5 | Talc | Smoothing | 1.65 | 2.45 | 3.25 |
| 6 | Purified water | Vehicle | Q. S | Q. S | Q. S |
| Enteric coated pellets weight | 179.19 | 198.15 | 213.4 | ||
Tab. 4. Enteric coating layer I on seal coated Pellets.
| Enteric layer-II coating | mg/capsule | |||||||
|---|---|---|---|---|---|---|---|---|
| S. no | Ingredients | Functional category | MEF-1 | MEF-2 | MEF-3 | MEF-4 | MEF-5 | MEF-6 |
| 1 | Selected enteric coating I pellets | 224.37 | 224.37 | 224.37 | 224.37 | 224.37 | 224.37 | |
| 2 | Eudragit L 100 | Delaying drug release | 5.61 | 11.22 | - | - | 11.22 | 2.24 |
| 3 | Eudragit S 100 | Delaying drug release | - | - | 5.61 | 11.22 | 2.24 | 11.22 |
| 4 | Triethyl citrate | Solubilizer, emulsifier | 0.375 | 0.75 | 0.375 | 0.75 | 0.937 | 0.937 |
| 5 | Talc | Smoothing | 0.75 | 1.50 | 0.75 | 1.50 | 1.875 | 1.875 |
| 6 | Purified water | Vehicle | Q. S | Q. S | Q. S | Q. S | Q. S | Q. S |
| 7 | Ethyl alcohol | Vehicle | Q. S | Q. S | Q. S | Q. S | Q. S | Q. S |
| Weight of final pellets | 231.10 | 237.84 | 231.10 | 237.84 | 240.64 | 240.64 | ||
Tab. 5. Enteric coating layer II on enteric coating layer I pellets.
Preparation of Extended Release (ER) pellets with drug loading, seal coating and enteric coatings
Drug loading: The formulation of Montelukast pellets were initiated with drug loading by Fluidized Bed Process (FBP). Three different formulations (MXR-1 to MXR-3) for drug loading on sugar spheres of size 20/25. All formulations were prepared by using equipment of fluid bed coater so the sugar spheres were kept in the fluid bed coater and drug loading can be done by MKS, Eudragit RSPO, triethyl citrate, talc dissolved in isopropanol and dichloromethane to make coating solution and coated on the sugar spheres. The pellets were filled in capsules. The formulations of drug loading illustrated in Table 6.
| Drug loading | mg/capsule | ||||
|---|---|---|---|---|---|
| S. no | Ingredients | Functional category | MXR 1 | MXR 2 | MXR 3 |
| 1 | Sugar spheres | 100 | 100 | 100 | |
| 2 | Montelukast sodium | API | 10 | 10 | 10 |
| 3 | Eudragit RSPO | Extended-release polymer | 5 | 7.5 | 10.00 |
| 4 | Triethyl citrate | Solubilizer, emulsifier | 1 | 1.50 | 2.00 |
| 5 | Talc | Smoothing | 1.50 | 2.25 | 3.00 |
| 6 | Isopropyl alcohol | Vehicle | Q. S | Q. S | Q. S |
| 7 | Dichloromethane | Vehicle | Q. S | Q. S | Q. S |
| Weight of pellets | 117.50 | 121.25 | 125.00 | ||
| Seal coating | |||||
| 8 | Drug loading pellets (weight of the pellets was adjusted based on the assay values) | 121.13 | 125.21 | 128.45 | |
| 9 | HPMC E5 | Binder | 8.47 | 8.76 | 8.99 |
| 10 | Talc | Smoothing | 0.94 | 0.97 | 1.00 |
| 11 | PEG 400 | Plasticizer | 0.94 | 0.97 | 1.00 |
| 12 | Purified water | Vehicle | Q. S | Q. S | Q. S |
| Final seal coated pellets weight | 131.48 | 135.91 | 139.44 | ||
Tab. 6. Formulations of drug loading and seal coating in ER Pellets.
Seal coating: The Seal coating was done by using HPMC E 5 with help of same FBP and illustrated in Table 6.
Enteric coatings-I and II: Pellets which are seal coated kept for the enteric coating with help of FBP in 02 layers. Layer-I was given with Eudragit L 30 D 55 (MXR-4 to MXR-6) and Layer-II with Eudragit L 100, Eudragit S 100 (MXR-7 to MXR-9). The prepared pellets were dried at 35°C upto 5-6 hrs for the complete evaporation of the moisture content. The pellets were filled into capsule shells, the weight of the pellets was adjusted based on the assay values for dissolution process. The formulations Enteric coatings were illustrated in Table 7.
| Enteric layer -1 | mg/capsule | ||||
|---|---|---|---|---|---|
| S. no | Ingredients | Functional category | MXR 4 | MXR 5 | MXR 6 |
| 1 | Drug loaded XR pellets | 131.48 | 135.91 | 139.44 | |
| 2 | Eudragit L 30 D 55 | Delaying drug release | 57.85 | 59.80 | 61.35 |
| 3 | Triethyl citrate | Solubilizer, emulsifier | 5.30 | 5.47 | 5.62 |
| 4 | Polysorbate 80 | Plasticizer | 0.97 | 1.00 | 1.03 |
| 5 | Talc | Smoothing | 2.91 | 3.00 | 3.09 |
| 6 | Purified water | Vehicle | Q. S | Q. S | Q. S |
| Enteric coated pellets weight | 198.51 | 205.18 | 210.53 | ||
| Enteric layer-II | MXR 7 | MXR 8 | MXR 9 | ||
| 7 | Enteric coated pellets layer-1 | 198.51 | 205.18 | 210.53 | |
| 8 | Eudragit S 100 | Delaying drug release | 9.92 | 10.25 | 10.52 |
| 9 | Eudragit L 100 | Delaying drug release | 1.98 | 2.05 | 2.10 |
| 10 | Triethyl citrate | Solubilizer, emulsifier | 0.83 | 0.86 | 0.88 |
| 11 | Talc | Smoothing | 1.67 | 1.73 | 1.78 |
| 12 | Purified water | Vehicle | Q. S | Q. S | Q. S |
| 13 | Ethyl alcohol | Vehicle | Q. S | Q. S | Q. S |
| Weight of final pellets | 212.91 | 220.07 | 225.81 | ||
Tab. 7. Formulations of enteric coatings on seal coated pellets.
Evaluation of formulations
UV analytical method: Standard plot of Montelukast sodium: The primary stock solution of montelukast sodium (10 mg in 100 mL methanol) was prepared with concentration of 100 μg/ml. Timely Aliquots of stock solution were pipetted and kept into 10 ml volumetric flask, volume was adjusted up to 10 ml to obtain concentrations of 2-14 μg/ml with hydrochloric acid buffer pH 1.2 and obtain concentration of 2-20 μg/ml with phosphate buffer pH 6.8. The absorbance was observed at λmax versus respective blank buffer solution. The calibration curves were plotted. The λmax was found to be 345 nm in phosphate buffer, pH of 6.8. The shift in λmax to 385 nm was observed in acidic media (0.1 N HCl) due to fluorescent properties of Montelukast sodium and also the working standard solutions of Montelukast sodium in 0.5% SLS in water were separately scanned for λmax in UV visible spectrophotometer 285 nm (in 900 ml of water having 0.5 % SLS) [12-15].
Drug excipient compatibility study: Differential Scanning Calorimetry (DSC) as well as Fourier-Transformed Infrared Spectroscopy (FTIR) was used to assess drug with Selected excipients compatibility. Using a BRUKER FTIR IFS 68 Spectrophotometer, FTIR was done across a wavelength between 4000 cm-1-400 cm-1.Sample is incorporated with KBr and pressed into discs using hydraulic press for 5 minutes under 5 tons of pressure, the pellet was analysed to obtain the spectrum [12,16,17].
A DSC-60 calorimeter of Shimadzu, Tokyo, Japan was used to per form DSC. The samples were kept in a 40 μl aluminum pan with thickness of 0.1 mm. The pan is then sealed by aluminum perfo rated cover and heated up to 250°C using nitrogen flow at a rate of 5°C min [16-18].
Characterization of Montelukast sodium pellets
Bulk density: Weighed required amount of prepared pellets were placed in 100 mL measuring cylinder and measure the occupied volume. Then the bulk density is determined using following cal culation [19].
Bulk density=Weight of the pellets/Bulk volume
Drug content: Pellets were grounded by placing in a mortar pestle and 10mg kept into volumetric flask of 50 mL, dissolved in 5 ml of methanol/ethanol and sonicate for 5 min. Finally, makeup the solution of phosphate buffer pH of 6.8, shake thoroughly the solution prepared. The residue has been separated using clarification process using Whattman’s filter paper grade No. 41. Dilutions were made and absorbance was measured at 345 nm for diluted solutions [13,19].
Scanning Electron Microscopy (SEM): The physicochemical properties as well as biopharmaceutical properties for finished dosage forms may be affected by the particles size, shape of excipient. In formulation development, parameters play a critical role are effect of the shape on flow, stability, mixing efficiency, dissolution and on formulation homogeneity. The surface morphology has been determined by scanning electron microscope (JEOL 5400, Japan) for visualization of surface [19,20].
In vitro dissolution studies: USP type I dissolution of Basket type was used (LABINDIA DS 8000, India) and the study was performed at 37°C ± 0.5 C at speed of 50 rpm. The in vitro dissolution of drug loaded pellets in 0.5% SLS in water, enteric coated pellets in capsule have been performed in 0.1 N HCl with Tween 80 in 2 h (stated as average gastric emptying time) and in pH of 6.8 phosphate buffer with Tween 80 for remained hours (stated as average transit time of small intestine). The pellets placed in capsule, for IR pellets dissolution studies done by using Phosphate buffer pH 6.8. For Enteric coated pellets in capsule of IR, ER dissolution study performed by first 2 hr with 500 ml of 0.1 N HCl with Tween 80 and followed by phosphate buffer of pH 6.8 with Tween 80. The aliquots were taken at specified time intervals and each 5 ml of sample was replaced by freshly prepared medium. The prepared samples were determined using UV Visible spectrophotometer of λ-max at 345 nm using phosphate buffer pH 6.8 and also using 385 nm in acidic media 0.1 N HCl [13].
Stability studies: The optimized formulations of Enteric coated IR, ER pellets were selected, filled, sealed in 100 CC HDPE con tainer. Three sets of each formulation were prepared and stored at kept in stability chamber for six months at 40°C ± 2°C/70% ± 5% RH. The samples were evaluated at 1, 3 and 6 months for colour change, drug content and drug release [21,22].
Microscopic analysis: Photographs were collected at 40X optical magnification and a microscopic analysis of Montelukast sodium IR and ER Pellets was conducted. The QUASMO ANISO 9001 2000 microscope type, which has a digital camera DCE2.17, is the one used to examine the morphological features [23].
Results and Discussion
Evaluation test investigations were performed on prepared formulations and the outcomes were deemed satisfactory. The Bulk density was within the limits of 0.486 ± 0.01-0.615 ± 0.009 g/ sq.cm.
Drug content: The drug content of IR Pellets of MEF-1 to MEF 6 within the range of 98.18 ± 0.11-99.01 ± 0.33 and MXR-7 to MXR-9 showed 97.99±0.19-98.15 ± 0.21.
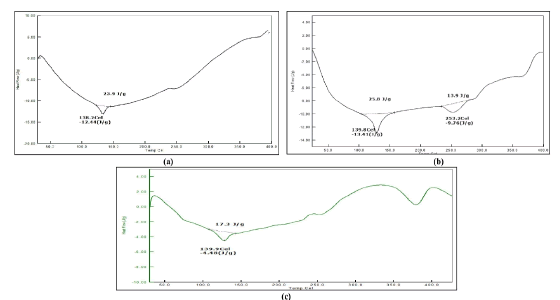
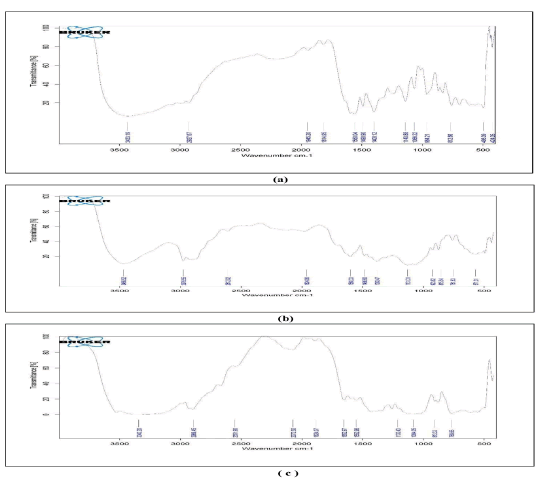
Drug-excipient compatibility: The optimized formulations were assessed using FTIR and DSC; The results obtained shown that no chemical interactions among the drug and incorporated excipients. T he results illustrated in Figures 1 and 2.
Fig. 1. DSC thermogram of (a) Montelukast Sodium (MKS); (b) MKS+Eudragit L30 D 55+Eudragit L100 and S 100; (c) DSC thermogram of MKS+Eudragit RSPO+Eudragit L100 and S 100.
Fig. 2. FTIR spectra of (a) Montelukast Sodium (MKS); (b) MKS+Eudragit L30 D 55+Eudragit L100 and S 100; (c) MKS+Eudragit RSPO+Eudragit L100 and S 100.
In vitro dissolution studies
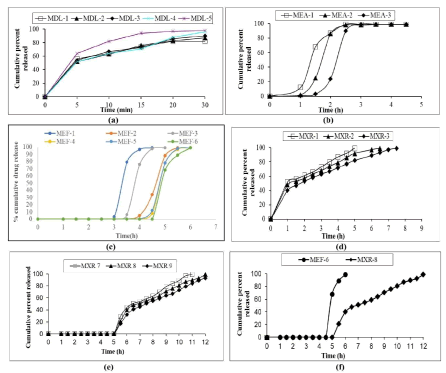
Dissolution result showed 98% of drug release within 30 min for MDL-5. After it has been coated with seal and enteric coatings of MEF-1 to MEF-6 showed 99% of drug release with lag time but optimised MEF-6 showed 99% of drug release in 6 h with 4.5 h lag time compared to remaining formulations. Similarly enteric coated ER pellets of MXR-7 to MXR-9 showed extended drug release after the lag time, MXR-8 showed 99% of drug release at 12 h as extended manner with 5 h lag time. The results illustrated in Figure 3.
Fig. 3. Dissolution profile plot of (a) MKS drug loaded pellets; (b) MKS enteric coating-I of IR pellets; (c) MKS IR pellets after Enteric Coatings; (d) MKS ER drug loaded pellets with seal coating; (e) MKS ER pellets after enteric coatings; (f) comparison of MKS optimised IR and ER pellet.
Scanning Electron Microscopy (SEM) of pellets: SEM showed the surface morphology of coated pellets with uniform surface. The SEM images illustrated in Figure 4.
Microscopic analysis: Montelukast sodium IR, ER Pellets were examined under a microscope and photomicrograph were depicted in Figure 5.
Fig. 4. SEM of Montelukast sodium optimised IR, ER pellet.
Fig. 5. Photomicrographs of Montelukast sodium pellets.
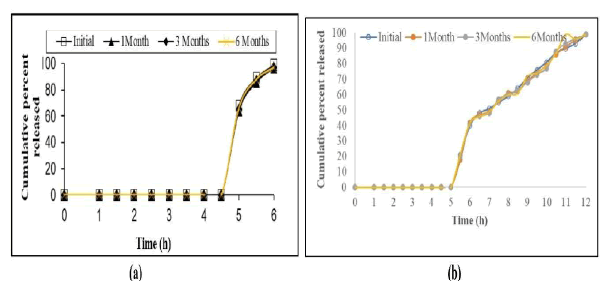
Stability studies: Stability Studies were performed for optimised formulations of MEF-6 and MXR-8 was stable as evidenced by the absence of deviations in drug content %, colour change and drug release. The results illustrated in Figure 6.
Fig. 6. Stability study of optimised pellets (a) MEF-6; (b) MXR-8.
Conclusion
Utilising an already available active component (Montelukast sodium), a good trial was constructed an efficient pulsatile drug delivery systems pellets prepared by FBP with enteric coatings designed as Immediate Release (IR) as well as Extended Release (ER). The IR pellets were designed in such a way for drug release immediately after the lag time. The ER pellets were designed that the drug release at an extended rate after a lag time. So, the enteric coating on Montelukast sodium of IR, ER pellets given by the selected polymers Eudragit L 30 D 55, Eudragit L100 and Eudragit S100 showed release of drug occurs after the lag time. The optimised formulations of MEF-6 and MXR-8 showed 99% of drug release after the lag time. The characterization and compatibility study of the designed system reveals that prepared formulations of drug stability. Thus, it can be concluded from the above results that the polymers listed above can be used to formulate a pulsatile drug delivery system for enteric coated Montelukast sodium pellets and this type of drug release which is modified used for the therapy of the asthma attacks especially during dreadful diseases like non small lung cancers, other pulmonary disorders where exacerbation was observed in early hours at morning.
Funding
There is no funding source.
Author’s Contributions
PS: Study design, methodology, collecting the data, analyzing the results, investigation and writing original manuscript under guidance of supervisor. LS: Conceptualization, supervision. All authors reviewed results and approved the final version of manuscript.
Conflicts of Interest
None to declare.
References
- Sowmya P, Dp VE, Nayek S. Pulsatile drug delivery system: a formulation approach for treatment of diseases. Int J Curr Pharmaceut Res. 2020; 12:16-21.
- Singh A, Dubey H, Shukla I, Singh DP. Pulsatile drug delivery system: an approach of medication according to circadian rhythm. J Appl Pharm Sci. 2012; 02:166-176.
- Anusha V, Umashankar MS, Kumar YG. Pulsatile drug delivery system-an innovative method to treat chronotherapeutic diseases by synchronizing drug delivery with circadian rhythm. J Appl Pharm Sci. 2023; 13:66-78.
- Bodke V, Tekade BW, Badekar R, Phalak SD, Kale M. Pulsatile drug delivery systems the novel approach. Int J Pharm Pharm Sci. 2024; 16:1-11.
- Kumar R, Chandra A, Saloni S, Gautam PK. Advanced multiple unit controlled release floating beads: A review. World J Pharm Res. 2017; 6:238-259.
- Sivalingan G, GNK G, Chandrasekaran M. Multiparticulate Drug Delivery System. Res J Pharm Technol. 2020; 13:3501-3507.
- Majeed SM, Al-Shaheen MK, Al-Zidan RN, Mahmood SM. Multiple Unite Pellet Systems (MUPS) as drug delivery model. J Drug Deliv Ther. 2020; 10:231-235.
- Deb R, Ahmed AB. Pellets and pelletization techniques: A critical review. Int Res J Pharm. 2016; 4:90-95.
- Lane SJ. Leukotriene antagonism in asthma and rhinitis. Respiratory Med. 1998; 92:795-809.
[Crossref] [Google Scholar] [PubMed]
- Lu CY, Zhang F, Lakoma MD, Butler MG, Fung V, et al. Asthma treatments and mental health visits after a Food and Drug Administration label change for leukotriene inhibitors. Clin Ther. 2015; 37:1280-1291.
[Crossref] [Google Scholar] [PubMed]
- Krishna MR, Rani AP, Saikishore V. Preparation and evaluation of pulsatile drug delivery of montelukast sodium. Indo Am J Pharm Sci. 2017; 4:1855-1861.
- Diggikar SS, Nandgude TD, Poddar SS. Formulation of modified release pellets of Montelukast sodium. Res J Pharm Technol. 2018; 11:31-37.
- Ranjan OP, Nayak UY, Reddy MS, Dengale SJ, Musmade PB, et al. Osmotically controlled pulsatile release capsule of montelukast sodium for chronotherapy: Statistical optimization, in vitro and in vivo evaluation. Drug Deliv. 2014; 21:509-518.
[Crossref] [Google Scholar] [PubMed]
- Patel R, Solanki R, Shaikh Z, Chauhan S, Parikh S, et al. Development of in vitro dissolution test method for bilastine and montelukast fixed-dose combination tablets. Dissolution Technol. 2023; 30:246-250.
- Shrishailappa DD, Tikare N, Halakatti P, Desai A, Muchandi I. Formulation and Evaluation of Pulsatile Drug Delivery System of Montelukast Sodium. RGUHS J Pharm Sci. 2018; 8.
- Neeharika MS, Jyothi BJ. Preparation and evaluation of zafirlukast compression coated tablets for chronotherapeutic drug delivery. J Pharm Res Int. 2021; 33:154-166.
- KRISHNA NS, Jayanthi B, Madhukar A. Formulation development and evaluation of chronomodulated drug delivery system by zafirlukast. Int J App Pharm. 2021; 13:211-220.
- Elmubarak EH, Osman ZA, Abdelrahman MO. Formulation and evaluation of solid dispersion tablets of furosemide using polyvinylpyrrolidone K-30. Int J Curr Pharm Res. 2021; 13:43-50.
- Matkovic SR, Valle GM, Briand LE. Quantitative analysis of ibuprofen in pharmaceutical formulations through FTIR spectroscopy. Lat Am Appl Res. 2005; 35:189-195.
- Sopanrao Muley S, Nandgude T, Poddar S. Formulation and optimization of lansoprazole pellets using factorial design prepared by extrusion-spheronization technique using carboxymethyl tamarind kernel powder. Recent Pat Drug Deliv Formul. 2017; 11:54-66.
[Crossref] [Google Scholar] [PubMed]
- Kulkarni U, Rao NR, Patil BS. Fabrication and evaluation of fast dissolving tablets of Tizanidine hydrochloride by solid dispersion technique. Int J Pharm Sci Rev Res. 2011; 6:88-93.
- TürkoÄlu M, Varol H, Celikok M. Tableting and stability evaluation of enteric-coated omeprazole pellets. Eur J Pharm Biopharm. 2004; 57:279-286.
[Crossref] [Google Scholar] [PubMed]
- Raju PN, Prakash K, et al. Preparation of zidovudine extended release matrix tablets with various controlled release polymers: A feasibility study of granulation and compression. International Int J Pharm Sci Nanotechnol. 2011; 3:1230-1239.