Research Article - Onkologia i Radioterapia ( 2022) Volume 0, Issue 0
44/47 Scandium labelled cholecystokinin derivative for cancer theragnostics
Eun-ha Cho*, Jae-cheong Lim, So-young Lee and Ul-jae ParkEun-ha Cho, Radioisotope Research Division, Quantum and Convergence Sciences, Korea Atomic Energy Research Institute, 111, Daedeok-daero989beon-gil, Yuseong- gu, Daejeon, Republic of Korea; ADDRESS: 111, Daedeok-daero989beon-gil, Yuseong-gu, Daejeon 34057, Postcode: 340571, Republic of Korea, Email: choeh36@kaeri.re.kr
Received: 07-Feb-2022, Manuscript No. OAR-22-53819; , Pre QC No. OAR-22-53819 (PQ); Editor assigned: 11-Feb-2022, Pre QC No. OAR-22-53819 (PQ); Reviewed: 25-Feb-2022, QC No. OAR-22-53819; Revised: 04-Mar-2022, Manuscript No. OAR-22-53819; Published: 11-Mar-2022, DOI: 10.4172/1896-8961.16.S1.001
Abstract
The Cholecystokinin (CCK) receptors are known to overexpress in various types of tumors. Through a previous study, a cyclic CCK analogue, DOTA-[Nle]-cCCK, was confirmed to have high in vivo stability and the tumor target ability of DOTA-[Nle]- cCCK capable of binding to the CCK receptor was confirmed through Lu-177 labeling. In this study, DOTA-[Nle]-cCCK was labeled with the pair-isotope, Sc-44/47, to confirm a technology that possibly could be applicable to radiopharmaceutical. First, we confirmed that the CCK receptor was overexpressed in AR42J, a cancer cell overexpressed in cancer tissue, and measured the binding ability of the receptor and DOTA-[Nle]-cCCK. We established the labeling method of radioactive scandium, and we confirmed that the Sc-44 labeled DOTA-[Nle]-cCCK administered to mice remained mostly in the bladder within an hour. Cell experiments with Sc-47 labeled DOTA-[Nle]-cCCK confirmed that more than half of the cancer cells were killed at a concentration of 5 MBq/ml. Through this study, we were able to confirm the diagnostic/therapeutic applicability of the DOTA-[Nle]-cCCK label with pair-isotope Sc-44/47.
Keywords
Theragnostic nuclides, antibody, peptides, Sc-47, gamma rays
Introduction
Targeted radionuclide therapy using radioisotopes is emerging as a very important technology in diagnosing and treating cancer in the field of cancer therapy [1]. There are several methods for using targeted radionuclide therapy, but they are largely classified into two methods, using antibodies or using peptides, depending on the type of carrier [2]. Antibodies are protein-type substances and macromolecules with a very large structure. Therefore, structural denaturation easily occurs; handling conditions are very difficult, and the price is high. However, when trying to increase the integration rate for a specific target, there is the advantage of high specificity. On the other hand, peptides have a relatively small structure, and the synthesis method is simple; therefore, they are easy to handle, and their price is low [3]. Because they have a low molecular weight structure compared to antibodies, the binding specificity of peptides with a specific target may be small; however, depending on the selection of the target, effective results may be produced at a lower cost than with an antibody. Generally, the carrier material labelled with the radioisotope is a combination of a material capable of binding to a specific target in the body and a chelator [4]. Many studies have recently been done on radioactive isotopes such as Lu-177, called theragnostic nuclides, because they can simultaneously emit beta and gamma rays to perform treatment and diagnosis at the same time [5]. The use of a pair-isotope is a way to replace and compensate for the defects of the theragnostics, and the representative pair-isotope is Scandium-44/47 [6]. The two elements have the same chemical properties, but they emit radiation with different properties because the physical properties differ depending on the difference in mass number. In the case of Sc-47, radiation that is good for treatment is generally emitted; thus, it can be used as a therapeutic radioisotope, and in the case of Sc-44, radiation that is good for diagnosis is released; therefore, it can be used as a diagnostic radioisotope [7]. Theragnostic nuclides are nuclides that are attracting attention because one nuclide can be used for both diagnosis and treatment at the same time, but it is impossible to completely replace the pair-isotope if the diagnosis and treatment need to be reliably separated into distinct stages.
CCK receptors are overexpressed in several cancer cells and have a high expression rate, especially in lung cancer and pancreatic cancer [8]. CCK receptors are pharmacologically divided into two main categories depending on their suitability with gastrin. The suitability of the CCK1 receptor is low, but the CCK2 receptor exhibits a high binding strength due to its high suitability with gastrin [9]. In CCK receptors, Tyr residues have a great influence on determining binding ability. When the Tyr residue is sulfurized, the bonding strength with the peptide can be maintained very high [10]. In the case of CCK binding to the CCK receptor, the amino acid residues in the C-terminal are used for binding to the receptor [11]. The C-terminal consists of Trp-Met-Asp-Phe-NH2, and the synthesis of CCK-like peptides using these amino acid sequences results in a material that can bind specifically to CCK receptors. In addition, by combining substances that can be labeled with radioisotopes to the N-terminal part, it is possible to synthesize substances that can be used in the development of labeling drugs targeting CCK receptors using radioisotopes.
Materials and Methods
Chemicals
RPMI-1640 media were purchased from LONZA (MD, Walkersville, USA). DyLight 488 NHS Ester was purchased from Thermo Scientific (NH, Hudson, USA). Fetal bovine Serum (FBS), penicillin, streptomycin, L-glutamine, 0.25% trypsin-EDTA, Dimethyl Sulfoxide (DMSO), phosphate-buffing saline, formaldehyde (CH2O), sucrose, propidium iodide and crystal violet dyes were purchased from Sigma-Aldrich Co., Ltd., Louis St.
Cells
AR42J pancreatic cancer cells were purchased from ATCC (Manassas, VA, USA) and cultured in RPMI-1640, supplemented on a 100 mm plate with 100 units/mL penicillin, 100 g/mL streptomycin and 10% fetal bovine serum. In addition, AR42J pancreatic cancer cells were cultured at 37°C for fusion of up to 90% in a 5% CO2 atmosphere in air.
Animals
Male C57BL/6 mice (18-22 g, 6 weeks) were supplied by Nara Biotec Inc. (Seoul, Korea). All animals were kept in wire cages at 20 and 22°Cand given standard laboratory chow and water ad libitum, and the humidity was maintained at 50 ± 10 %. The management protocols and test system and animal care were authorized by the committee of Korea Atomic Energy Research Institute. All experiments were conducted in accordance with the relevant guidelines and regulations.
Peptide
The DOTA-[Nle]-cCCK was synthesized by the automated Multiple Biomolecular Synthesizing System from Peptron, Daejeon, South Korea (Figure 1). Fmoc-amino acid bond 4-Methylbenzhydrylamine (MBHA) resin was used as a fixed polymer support for solid phase synthesis. The Fmoc protector was removed from the resin boundary Fmoc-Met-OH under standard cutting conditions, and as a result, the peptide was separated from the polymer support by mixing with 90% TFA containing 2.5% Triisopropylsilane (TIS), 2.5% Ethianisol (EDT), 2.5% thionisol and 2.5% deionized water [12].
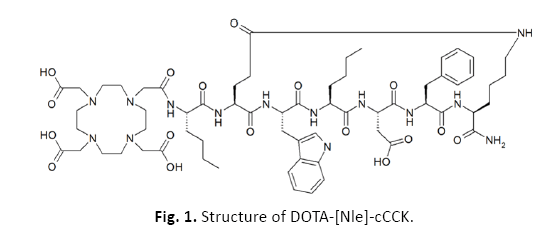
Figure 1: Structure of DOTA-[Nle]-cCCK.
Combining receptors
AR42J cells were cultured in cover glass-bottom plate for the analysis of binding a residual amount of DOTA-[Nle]-cCCK. AR42J cells were treated for 20 minutes with 10 mL of DyLight 488 NHS Ester or DyLight 488 NHS Ester-conjugated DOTA-[Nle]-cCCK, and propidium iodide was added for nuclear detection. The cells were fixed with 4% CH2O and 3% sucrose. Fluorescent microscopy (Leica Microsystems, Wetzlar, Germany) was used to observe the stained cells.
Radiolabeling
DOTA-[Nle]-cCCK was liquefied with a sodium acetate buffer (50 mM, pH 5.5) to give 10-6 mol/mL. The final volume was 1 mL by injecting a diluted 44ScCl3 or 47ScCl3 solution (37 MBq) of 5.5 M HCl into the vial of the peptide (10-8 mol) solution. Then, the bottle was heated at 90°C for 20 minutes. Radioactive labeling yield, radioactive chemical purity, and stability of the radioactive labeling compounds were analyzed by a water chromatograph equipped with an X-Terra C-18 column. A binary gradient system with a flow rate of 1.0 mL/min was dissolved using a 0.1% elution solvent, 5% acetonitrile, 0.1% acetonitrile, and 5% acetonitrile.
In vivo excretion
The mice were anesthetized by exposure to 2% isoflurane with oxygen. Small animal PET images were taken in the mice with Sc-44, Sc-44-DOTA and Sc-44-DOTA-[Nle]-cCCK at intervals of 1 hour and 17 hours after intravenous injection through the tail veins.
Cytotoxicity
AR42J cells were plated on to a 12-well culture plate with 1 ml of RPMI-1640 growth medium at a density of 1 × 105 and cultured at 37 °C and 5% CO2 for 24 hours in a humidified atmosphere of 95% air. The growth medium was replaced by FBS containing a phenol red pre-experimental medium and 10 μl of DMSO. The treatment lasted 24 hours. At the end of the culture, the cytotoxicity of the compound was evaluated by a dye absorption test using crystal violet as described above [13]. Optical density measurements were obtained at 540 nm using a microplate reader. The average absorbance value of the control device was considered as 100% cell viability.
Statistical analysis
All values are expressed as the mean ± Standard Deviation (SD). Student’s t-test was used to evaluate differences between groups and the two-sided Spearman’s correlation coefficient was used to determine the relationship between variables. A probability of less than 0.05 was considered to be statistically significant.
Results
We found from immune-histogram images that many CCKRs were stained in lung cancer tissues, but there was little expression of CCKR in normal lung tissues (Figure 2). From this result, it was confirmed that CCKR, which was confirmed to be overexpressed only in cancer tissue, was a suitable target for use in this experiment.
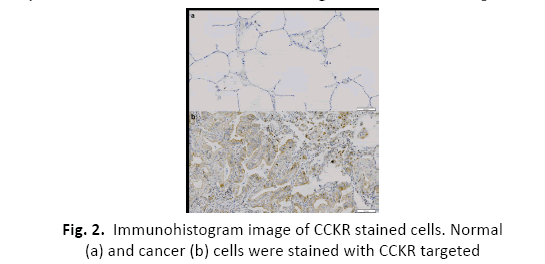
Figure 2: Immunohistogram image of CCKR stained cells. Normal (a) and cancer (b) cells were stained with CCKR targeted
In Figure 3, red staining indicates the cell nucleus, and both the “NORMAL” and the “SAMPLE” groups have red stained nuclei, indicating that actual cells exist. However, in the case of the green-looking DyLight 488 NHS ester, a large amount remained after washing in the “SAMPLE” group treated with DOTA-[Nle]- cCCK, while little remained after washing in the “NORMAL” group treated only with the DyLight 488 NHS ester. From this experiment, it was confirmed that DOTA-[Nle]-cCCK binds well to the cancer cell AR42J, and even when applied to actual treatment, it was expected to bind to cancer cells and have a role in killing cancer cells.
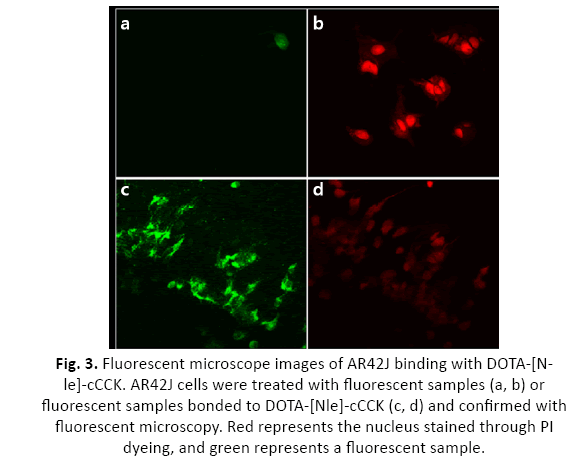
Figure 3: Fluorescent microscope images of AR42J binding with DOTA-[Nle]- cCCK. AR42J cells were treated with fluorescent samples (a, b) or fluorescent samples bonded to DOTA-[Nle]-cCCK (c, d) and confirmed with fluorescent microscopy. Red represents the nucleus stained through PI dyeing, and green represents a fluorescent sample.
A labeling experiment was done to check whether DOTA-[Nle]- cCCK is labeled with Sc-44. From this experiment, it was possible to confirm the labeling rate and establish a stable labeling protocol. After the labeling, we conducted ITLC using Na-citrate deployment solvent for free Sc-44, Sc-44-DOTA and Sc-44-DOTA-[ Nle]-cCCK. As a result of the experiment, it was confirmed that one peak came out neatly for each group, and the location of each peak was different. As a result of the ITLC measurements, free Sc-44 was measured at 78 mm; Sc-44-DOTA was measured at 43 mm, and Sc-44-DOTA-[Nle]-cCCK was measured at 21 mm. When the Na-citrate buffer was used as a deployment solvent, the affinity with the deployment solvent was high in the order of free Sc-44, Sc-44-DOTA, and Sc-44-DOTA-[Nle]-cCCK, and through this method, it was possible to check whether it was labeled (Figure 4).
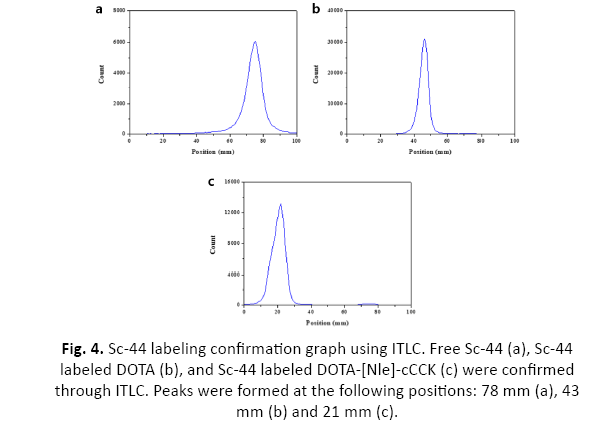
Figure 4: Sc-44 labeling confirmation graph using ITLC. Free Sc-44 (a), Sc-44 labeled DOTA (b), and Sc-44 labeled DOTA-[Nle]-cCCK (c) were confirmed through ITLC. Peaks were formed at the following positions: 78 mm (a), 43 mm (b) and 21 mm (c).
To measure in vivo excretion, free Sc-44, Sc-44-DOTA, and Sc-44-DOTA-[Nle]-cCCK were injected into the mouse at a concentration of 0.045 mCi/100 μL, respectively. The mouse was anesthetized using isoflurane, and PET imaging was performed immediately after injection, 1 hour after injection, and 17 hours after injection. From the PET imaging results, unlike free Sc-44, it was confirmed that only a little residual amount of Sc-44-DOTA and Sc-44-DOTA-[Nle]-cCCK remained in the body other than in the bladder after 1 hour. To have high value as a radioactive drug, substances that are not bound to the target must be excreted quickly. Through this experiment, high excretion rates were confirmed, and in the case of Sc-44-DOTA-[Nle]-cCCK, unlike Sc-44-DOTA, the possibility as a radiopharmaceutical could be confirmed because target accumulation in the body can be expected through the peptide (Figure 5).
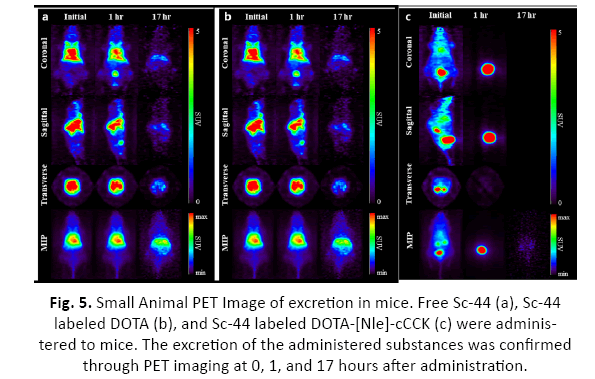
Figure 5: Small Animal PET Image of excretion in mice. Free Sc-44 (a), Sc-44 labeled DOTA (b), and Sc-44 labeled DOTA-[Nle]-cCCK (c) were administered to mice. The excretion of the administered substances was confirmed through PET imaging at 0, 1, and 17 hours after administration.
Cell viability experiments of Sc-47-DOTA-[Nle]-cCCK to confirm its cancer treatment capacity showed that almost half of the cancer cells were killed when treated with 5 MBq/ml of Sc-47-DOTA-[ Nle]-cCCK. This result is similar to the values derived from the experiment using radioisotopes for the treatment, and it was confirmed that there is a possibility that the Sc-47-DOTA-[Nle]-cCCK can be used as an actual therapeutic agent (Figure 6).
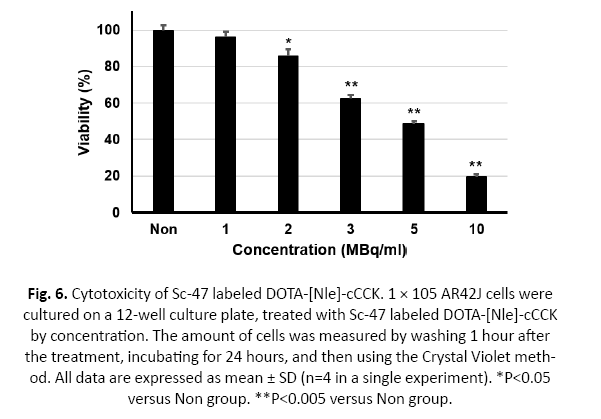
Figure 6: Cytotoxicity of Sc-47 labeled DOTA-[Nle]-cCCK. 1 × 105 AR42J cells were cultured on a 12-well culture plate, treated with Sc-47 labeled DOTA-[Nle]-cCCK by concentration. The amount of cells was measured by washing 1 hour after the treatment, incubating for 24 hours, and then using the Crystal Violet method. All data are expressed as mean ± SD (n=4 in a single experiment). *P<0.05 versus Non group. **P<0.005 versus Non group.
Discussion
Using radiopharmaceuticals that emit radiation have many difficulties. First, to produce radiopharmaceuticals, a separate production facility capable of handling radiation must be secured to proceed with the production process placing workers at risk of exposure. When using radiation therapy, it is necessary to secure separate isolation beds in hospitals, and only those with specific qualifications can handle radiopharmaceuticals [14]. In addition, related wastes generated during production and use should be treated as radioactive waste. To proceed with this process, it takes a lot of money and requires various procedures. Therefore, radiopharmaceuticals are inevitably avoided if there are other pharmaceuticals that can replace them.
However, the reason why radiopharmaceuticals are still used is that their effectiveness, which cannot be replaced by other pharmaceuticals, can be expected. Radiation emitted by radioisotopes is a characteristic of radiopharmaceuticals that is difficult to artificially produce in other alternative pharmaceuticals, and radiopharmaceuticals that effectively utilize radiation are useful [15-17].
Recently, research has been actively conducted on the development of radiopharmaceuticals using theragnostic radioisotopes that can be applied simultaneously for diagnostic and treatment purposes by simultaneously emitting beta and gamma rays [18-20]. A pair-isotope is a more customized way to use theragnostic radioisotopes, which are applied simultaneously as one nuclide for diagnosis and treatment [21]. Even if the dose is adjusted to use the drug labeled with the theragnostic radioisotopes only for diagnosis, the effect of beta-ray emission on the body cannot be completely prevented. However, when a pair-isotope is used, radioactive drugs with the same chemical properties but different types of radiation emitted can be developed. For diagnosis, gamma-ray-emitting radioisotopes can be administered to block unnecessary in vivo effects by the beta- rays, and for treatment, beta-ray-emitting radioisotopes can be selected and administered, and if both are administered at the same time, it is possible to confirm the movement in vivo. Considering the characteristics of radioisotopes, Sc-44 and 47 are a suitable pair-isotope for medical applications [7].
Conclusion
The aim of this study was to confirm the medical applicability of labeling Sc-44/47, a pair-isotope, on a carrier compound derived in a previous study and to confirm the movement in vivo and its ability as a cancer therapy. The carrier compound was prepared targeting CCKR, which was confirmed to have increased expression in cancer cells, and its binding ability with the receptors was confirmed following previous studies. By standardizing the labeling protocol of radioactive scandium, it was confirmed that it was stably labeled with a yield of 95% or more. In the case of radiopharmaceuticals, it is important that non-binding substances are excreted quickly without being accumulated in the liver or kidneys, and when Sc-44-DOTA-[Nle]-cCCK was administered to mice, it was confirmed that most of it gathered in the bladder within an hour. Finally, using Sc-47-DOTA-[Nle]-cCCK as a treatment, it was confirmed that AR42J cancer cells were killed, and the possibility of using it as a cancer treatment was also confirmed.
Funding
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2017M2A2A6A05016598).
Competing Interests
The authors declare no competing interests.
Data Availability
The data analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Contributions
EC conceived the idea and wrote the manuscript. JL, SL and UP searched the literature, collected the required contents. The final version of this manuscript was approved by all the authors.
References
- Goldsmith SJ. Targeted radionuclide therapy: A historical and personal review. Semin Nucl Med. 2020;50(1):87-97.
[Pubmed] [Crossref] [Google scholar]
- Ma L, Wang C, He Z, et al. Peptide-drug conjugate: a novel drug design approach. Curr Med Chem. 2017;24(31):3373-3396.
[Pubmed] [Crossref] [Google scholar]
- Mimmi S, Maisano D, Quinto I, et al. Phage display: an overview in context to drug discovery. Trends Pharmacol Sci. 2019; 40(2):87-91.
[Pubmed] [Crossref] [Google scholar]
- Kang L, Rosenkrans ZT, Cai W. 64Cu-labeled aptamers for tumor-targeted radionuclide delivery. Methods Mol Biol. 2019; 1974:223-231.
[Pubmed] [Crossref] [Google scholar]
- Li J, Oyen R, Verbruggen A, et al. Small Molecule Sequential Dual-Targeting Theragnostic Strategy (SMSDTTS): from Preclinical Experiments towards Possible Clinical Anticancer Applications. J Cancer. 2013;4(2):133-145.
[Pubmed] [Crossref] [Google scholar]
- Crawford JR, Robertson AKH, Yang H, et al. Evaluation of 209At as a theranostic isotope for 209At-radiopharmaceutical development using high-energy SPECT. Phys Med Biol. 2018;63(4):045025.
[Pubmed] [Crossref] [Google scholar]
- Müller C, Bunka M, Haller S, et al. Promising prospects for 44Sc-/47Sc-based theragnostics: application of 47Sc for radionuclide tumor therapy in mice. J Nucl Med. 2014;55(10):1658-1664.
[Pubmed] [Crossref] [Google scholar]
- Berna MJ, Tapia JA, Sancho V, et al. Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol. 2007;7(6):583-592.
[Pubmed] [Cross Ref] [Google scholar]
- Baldwin GS, Shulkes A. CCK receptors and cancer. Curr Top Med Chem. 2007;7(12):1232-8.
[Pubmed] [Crossref] [Google scholar]
- Ding XQ, Pinon DI, Furse KE, et al. Refinement of the conformation of a critical region of charge-charge interaction between cholecystokinin and its receptor. Mol Pharmacol. 2002;61(5):1041-1052.
[Pubmed] [Crossref] [Google scholar]
- Roosenburg S, Laverman P, van Delft FL, et al. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids. 2011;41(5):1049-1058.
[Pubmed] [Crossref] [Google scholar]
- Cho EH, Lim JC, Lee SY, et al. An assessment tumor targeting ability of 177Lu labeled cyclic CCK analogue peptide by binding with cholecystokinin receptor. J Pharmacol Sci. 2016;131(3):209-14.
[Pubmed] [Crossref] [Google scholar]
- Badisa RB, Tzakou O, Couladis M, et al. Cytotoxic activities of some Greek Labiatae herbs. Phytother Res. 2003;17: 472-476.
[Pubmed] [Cross ref] [Google scholar]
- Nishiyama H, Lukes SJ, Mayfield G, et al. Internal contamination of laboratory personnel by 131I1. Radiology. 1980;137(3):767-771.
[Pubmed] [Crossref] [Google scholar]
- Guerra Liberal FDC, O'Sullivan JM, McMahon SJ, et al. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother Radiopharm. 2020;35(6):404-417.
[Pubmed] [Cross Ref] [Google scholar]
- Kleynhans J, Grobler AF, Ebenhan T, et al. Radiopharmaceutical enhancement by drug delivery systems: A review. J Control Release. 2018;287:177-193.
[Pubmed] [Crossref] [Google scholar]
- Martiniova L, Palatis L, Etchebehere E, et al. Gallium-68 in Medical Imaging. Curr Radiopharm. 2016;9(3):187-207.
[Pubmed] [Crossref] [Google scholar]
- Baratto L, Duan H, Mäcke H, et al. Imaging the Distribution of Gastrin-Releasing Peptide Receptors in Cancer. J Nucl Med. 2020;61(6):792-798.
[Pubmed] [Crossref] [Google scholar]
- Cimini A, Ricci M, Chiaravalloti A, et al. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int J Mol Sci. 2020;21(11):3849.
[Pubmed] [Crossref] [Google scholar]
- Quintos-Meneses HA, Aranda-Lara L, Morales-Ávila E, et al. In vitro irradiation of doxorubicin with 18F-FDG Cerenkov radiation and its potential application as a theragnostic system. J Photochem Photobiol B. 2020;210:111961.
[Pubmed] [Crossref] [Google scholar]
- Li M, Sagastume EA, Lee D, et al. 203/212Pb Theranostic Radiopharmaceuticals for Image-guided Radionuclide Therapy for Cancer. Curr Med Chem. 2020;27(41):7003-7331.
[Pubmed] [Crossref] [Google scholar]



