Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 8
Protective effect of Sorafenib against 1,2-Dimethylhydrazine-induced colorectal cancer in Balb/c Mice
M. Al Hassan1, M. Al Battal2, J. Usta3 and J. Borjac1*2Faculty of Medicine, Department of Pathology, Beirut Arab University, Beirut, Lebanon
3Faculty of Medicine, Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut, Lebanon
J. Borjac, Faculty of Science, Department of Biological Sciences, Beirut Arab University, Debbieh, Lebanon, Email: MHassan@bac.edu.lb, j.borjac@bau.edu.lb
Received: 13-Jul-2021 Accepted: 19-Aug-2021 Published: 27-Aug-2021
Abstract
Colorectal Cancer (CRC) is the third leading cause of cancer death in the world whose incidence is progressively rising in developing nations. It is treated with both chemotherapeutic and immunotherapeutic agents. Sorafenib is a broad-spectrum multikinase inhibitor proven to be effective in treating different types of carcinoma, including liver, thyroid, renal, and breast cancers. This study aimed to investigate the effect of Sorafenib against 1,2-dimethylhydrazine (DMH)-induced CRC in mice. Mice were injected with DMH (20 mg/kg) over 12 weeks to induce the cancer. Mice were treated with Sorafenib (30 mg/Kg for 5 days). Colon tissues were examined by histological analysis, and gene expression level of key players involved in the Wnt signaling pathway were assessed using RT-PCR. Our results showed that Sorafenib restored the normal histology and structure of colonic tissue. Sorafenib downregulated the expression level of Wnt5a and Cyclin D1 and upregulated that of APC, GSK-3β and β catenin compared to untreated control. In conclusion, Sorafenib inhibits DMH-induced CRC by targeting the Wnt signaling pathway.
Keywords
DMH, colorectal cancer, Sorafenib
Introduction
Colorectal Cancer (CRC) is a common malignancy with high morbidity and mortality worldwide [1]. The incidence of new cases and mortality has been progressively declining for the past years, excluding younger adults (younger than 50 years), probably due to the increase in cancer screening and better therapeutic modalities [2]. CRC is the third most common cause of cancer death in both men and women in the United States, and it ranks second when men and women are combined [3].
Wnt ligands (Wnts) are secreted glycoproteins that activate the Wnt signaling pathway through the canonical or noncanonical pathway. Important players in the Wnt canonical pathway are the destruction complex components comprising of the Adenomatous Polyposis Coli (APC), casein kinase, axin, Glycogen Synthase Kinase (GSK3β), and β-catenin. In the canonical pathway and in the absence of the Wnt ligand, β-catenin is sequestered within the destruction complex, phosphorylated by GSK3β and tagged for proteosomal degradation. In the presence of Wnt, β-catenin is freed from the complex, and it is translocated to the nucleus where it interacts with T-cell factor (Tcf)/lymphoid enhancer factor 1 (Lef1) to regulate the transcription of diverse oncogenes. In fact, gain of function mutations in β-catenin gene are associated with the initiation and progression of various types of cancers, mainly CRC [4]. Likewise, loss of function mutations in the tumour suppressor genes GSK3β and APC are implicated in colorectal carcinogenesis. Moreover, studies have shown that Wnt inhibits apoptosis of colon Cancer Cell Lines (CCC) by blocking caspase-9 activation induced by chemotherapeutic drugs [1]. Consequently, various chemotherapeutic drugs have been synthesized to target this pathway in different solid tumors and hematological malignancies [5].
Sorafenib, a multikinase inhibitor, is capable of facilitating apoptosis, mitigating angiogenesis and suppressing tumour cell proliferation. Reported targets of Sorafenib include Vascular Endothelial Growth Factor Receptor (VEGFR), Raf family, and Platelet-Derived Growth Factor Receptor (PDGFR) belonging to the general class of tyrosine kinases [6]. Blocking growth signals in liver, thyroid, renal, and breast cancers underlie one of the key mechanisms by which Sorafenib exerts its antitumor effects leading to cell death. Previous studies have shown that Sorafenib inhibits the Wnt/β-catenin signaling in vitro in HepG2 hepatoma cells [7]. Furthermore, a study by [8]. Sorafenib was shown to alter the Wnt signaling by decreasing β-catenin protein levels in HepG2 (CTNNB1-class), SNU387 (Wnt-TGFβ-class), SNU398 (CTNNB1-mutation) and Huh7 (Lithium-chloride-pathway activation) cell lines. It was also able to attenuate the expression of liver-related Wnt-targets including GLUL, LGR5, and TBX3 [8].
Recently, a study by [9] revealed that Sorafenib potently suppresses the growth of colon cancer cells in human colon carcinoma cell line HCT116. In this context, the present study aims at expanding upon our previous in vitro study and providing a better understanding of the potential in vivo action of Sorafenib in a DMH-induced CRC model in mice.
Materials and Methods
Chemicals
DMH;1,2-Dimethylhydrazine dihydrochloride was obtained from ACROS Organics TM (Thermo Fisher Scientific, USA). Sorafenib Tosylate was purchased from Eton Bioscience Inc. (Catalogue Number 1100200013). All primers were purchased from Macrogen (South Korea), and fetal bovine serum (catalogue number F9665) and 2 mercaptoethanol (catalogue number M6250) were purchased from Sigma Aldrich (USA). The 100x penicillin-streptomycin solution (catalogue number L0022-100) was purchased from Biowest (USA). Direct-zol RNA Miniprep (Catalogue number: R2051) was purchased from Zymo Research (USA). QuantiTect® Reverse Transcription Kit (catalogue number: 205311) and QuantiFast® SYBR® Green PCR Kit (catalog # 204045) for real-time quantification of cDNA were obtained from QIAGEN® (USA). All other chemicals used were of high analytical grades.
Animal model
Six-week-old healthy female albino Balb/c mice were obtained from Beirut Arab University's animal facility (18 g-20 g each). Mice were placed under standard laboratory conditions of light (12-hours), temperature (22 ± 2) °C, and humidity set at standard animal house levels with ad libitum access to standard mouse diet and tap water. They were left to adapt under these conditions for one week prior to starting the experiments. Experimental procedures were carried out according to the approved guidelines of the Institutional Review Board (IRB) at Beirut Arab University.
Colon cancer induction
A total of 24 female Balb/c mice were used in this study. Mice were grouped 6 per cage. One group was used as normal control. The other mice groups were intraperitoneally injected with DMH at a dose of 20 mg/kg body weight once per week, over a period of 12 consecutive weeks to induce CRC. The experimental groups are shown in Table 1.
| Group A | Normal Control | Mice did not receive any treatment. This group served as normal control |
| Group B | DMH-injected | Mice received once per week an intraperitoneal injection of DMH dissolved in saline at a dose of 20 mg/Kg over 12 weeks to induce CRC |
| Group C | DMSO | Mice received DMSO (1% DMSO in PBS) by gavage for 5 consecutive days, 2 weeks after the final DMH exposure. DMSO was used as a vehicle to dissolve Sorafenib |
| Group D | Sorafenib Treatment | Mice received Sorafenib Tosylate treatment at 30 mg/kg by gavage for 5 consecutive days, 2 weeks after the final DMH exposure |
Tab. 1. Experimental design
Histological analysis
At the end of the treatment, the mice were fasted overnight, with only water access ad libitum. They were dissected under anaesthesia using diethyl ether. The entire large intestine from the ileocecal junction to the anal verge was excised, flushed with ice-cold PBS, and opened longitudinally. The number of colorectal polyps was counted by two independent observers. Part of the colorectal tissue was fixed in 10% formalin at room temperature for 24h and then sent to the Specialized Medical Laboratories (Beirut, Lebanon) for histological preparation and were stained using hematoxylin and eosin (H&E). Another part was directly stored at -80°C for molecular analysis.
Quantification of gene expression by RT-PCR
RNA extraction and quantification: Total RNA was extracted from colon homogenates using the Direct-zol RNA Miniprep according to the manufacturer’s protocol. Briefly, tissues were homogenized in 600 μl of TRI reagent and centrifuged at 16,000 g for 30 seconds to collect the supernatant. An equal volume of ethanol (95%-100%) was added to the supernatant and transferred into Zymo-Spin IIC Column with 400 μl RNA wash buffer in a collection tube. Further to centrifugation, 5 μl DNase I, 75 μl DNA Digestion Buffer were added to the column matrix and incubated at room temperature for 15 minutes. Further to incubation, 400 μl of Direct-zol Wash Buffer was added to the column and centrifuged to collect the pellet; this step was repeated twice. After that, 700 μl of RNA wash buffer was added to the column and centrifuged for 2 minutes. Then, column was transferred into an RNase-free tube where 50 μl of DNase/ RNase- Free water were added to the column matrix and centrifuged to elute the RNA. To check for the integrity of the eluted RNA, samples were electrophoretically separated on 1% agarose and visualized by UV illumination using ethidium bromide staining. RNA was quantified using DeNovix (Blue) DS-11 Spectrophotometer through its absorbance which was measured at 260 nm. Its purity was assessed from the 260/280 absorbance ratio.
cDNA synthesis: Reverse transcription was performed using the QuantiTect® Reverse Transcription Kit according to manufacturer’s protocol. Briefly, 1 μg of RNA samples were mixed with 3 μl gDNA Wipeout Buffer and 9 μL of RNase free water and incubated at 42°C for 2 minutes to effectively remove any contaminated genomic DNA in a total volume of 14 μl. Following genomic DNA removal, RNA samples were reverse transcribed using Quantiscript Reverse Transcriptase (1.5 μl), Quantiscript RT Buffer (6 μl), and RT Primer Mix (1.5 μl) at 42°C for 15 minutes in a final volume of 20 μl. The enzyme was then inactivated at 95°C for 3 minutes and the cDNA obtained was stored at -80°C for later use.
RT-PCR: The expression levels of Wnt signaling genes were quantified by quantitative real time polymerase chain reaction using QuantiFast® SYBR® Green PCR Kit which includes a two-step cycling protocol. First, 10 μL of 2x QuantiFast SYBR Green PCR Master Mix (containing HotStarTaq® Plus DNA Polymerase, QuantiFast SYBR Green PCR Buffer containing Tris·Cl, KCl, (NH4)2SO4, MgCl2, and additives as Q-Bond®, dNTP mix (dATP, dCTP, dGTP, dTTP), SYBR Green dye, and ROX™ passive reference dye), 2 μl of template cDNA, 2 μl of each primer (1 μM) and 6 μl RNase-free water were mixed in a final volume of 20 μl PCR reaction mixture. Second, cycling was carried out with a denaturation step at 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 10 s and annealing/extension at 57°C for 30 s. Each RT-PCR was performed in triplicate for RT-PCR yield validation. Primer sets used in this study are enlisted in Table 2. Measurement of gene expression was done by comparative threshold cycle (Ct) method with Glyceraldehyde-3 Phosphate Dehydrogenase (GAPDH) used as a reference gene. The mean Ct (mCt) values were determined for each gene. ΔCt value was established as the difference between the Ct of gene of interest and the Ct of GAPDH gene. Relative gene expression was calculated using the 2-ΔΔCT gene dosage ratio formula (GDR) where:
| Gene | Primer Sequence | Product size (bp) |
|---|---|---|
| Wnt5a | F: 5'-CTGGCAGGACTTTCTCAAGG-3' | 395 |
| R: 5'-CTCTAGCGTCCACGAACTCC-3' | ||
| GSK3β | F: 5'-TCCATTCCTTTGGAATCTGC-3' | 236 |
| R: 5'-CAATTCAGCCAACACACAGC-3' | ||
| APC | F: 5'-TGGAAGTGTGAAAGCATTGATGGAAT GTGC-3' | 348 |
| R: 5'-CCACATGCATTACTGACTATTGTCAAG-3' | ||
| β-catenin | F: 5'-GCTGACCTGATGGAGTTGGA-3' | 227 |
| R: 5'-GCTACTTGCTCTTGCGTGAA-3' | ||
| cyclin D1 | F: 5'-GGCACCTGGATTGTTCTGTT-3' | 232 |
| R: 5'-CAGCTTGCTAGGGAACTTGG-3' | ||
| GAPDH | F: 5’-TGGTGCTCAGTGTAGCCCAG-3’ | 111 |
| R: 5’-GGACCTGACCTGCCGTCTAG-3’ |
Tab. 2. Sequence of primers used in quantitative real-time PCR
ΔΔCt=(mCt gene of interest-mCt GAPDH) control sample- (mCt gene of interest-mCt GAPDH) test sample.
Statistical analysis
All statistical analyses were performed using Microsoft Excel and SPSS. They are shown as mean ± standard deviations. Statistical significance was assessed using One-way ANOVA test and by T-test. Graphs were drawn by GraphPad prism software and statistical significance was reported with a p-value<0.05 considered as significant.
Results and Discussion
Body weight changes
Upon monitoring body weight changes, it was noticed that DMH treatment (Group B), DMSO (Group C), and DMH mice treated with Sorafenib (Group D) showed significant reduction in body weight compared to normal control (Group A) (12.45 g, 11.80 g and 12.95 g versus 27.70 g, respectively p<0.001), as shown in Figure 1.
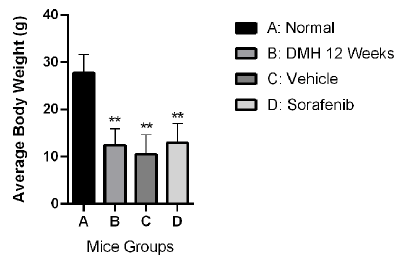
Figure 1: Effect of Sorafenib on body weight of DMH-injected Balb/c mice. Bars are represented as Means ± SD (n=3). Asterisks on bars represent significance relative to the control. (**) corresponds to p<0.01
However, treatment of DMH mice with Sorafenib (Group D) did not have any significant effect on bodyweight change compared to DMSO (Group C) or DMH groups (Group B).
Polyps count
As shown in Figure 2, mice receiving DMH for 12 weeks (Group B) and those receiving DMH and DMSO (Group C) developed 7.6 and 10 polyps respectively (p<0.001 and p<0.01) compared to control that showed none. However, Sorafenib treatment (Group D) induced a significant decrease by 60.52% in polyps count (3 polyps, p˂0.0001) compared to Group B, and by 70% in polyps count (p<0.0001) compared to Group C.
Figure 2: Polyps count in different DMH-injected Balb/c mice groups. Bars are represented as Means ± SD (n=3). Asterisks on bars represent significance relative to the control, and lines drawn upwards represent inter-categorical statistical significance. (***) and (****) correspond to p˂0.001 and p<0.0001
Histopathological analysis
Healthy control mice showed normal histological architecture of the colon with their straight crypts of Lieberkühn extending down to the muscularis mucosa with visible numerous goblet cells lining the crypts (Figure 3A). Macroscopic analysis showed the presence of polyps in the colons of untreated DMH mice and were confirmed by the histological analysis showing adenoma (Figure 3B). In this group, there were goblet cells depletion, high grade dysplasia, abnormal structures in the Lieberkühn glands, severe leukocyte infiltration to the lamina propria, and neoplastic cells. Likewise, colonic tissues from DMH-injected mice receiving DMSO (Group C), the vehicle used to dissolve Sorafenib, showed damaging effects similar to those seen in group B (Figure 3C). In contrast, upon the treatment of DMHinjected mice with Sorafenib, the normal architecture of the colon was restored, the size of colon adenomas was reduced, and goblet cells were regenerated with enhancement of the epithelial lining (Figures 3D).
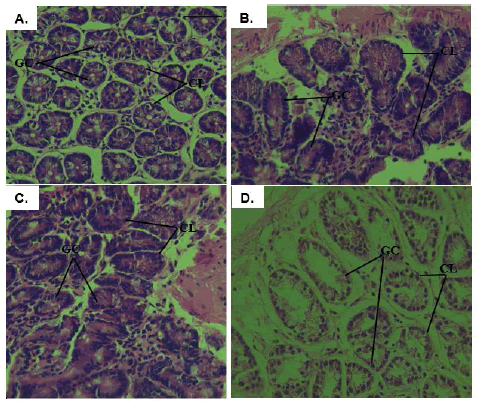
Figure 3: Histopathological analysis of colonic tissues from all experimental groups; A: photomicrographs of the colon of normal mice showing normal colonic architecture with Crypts of Lieberkühn (CL) and many goblet cells (GC) (H&E × 40); B and C: colon tissues isolated from DMH mice and vehicle treated DMH mice showing high grade dysplasia, abnormal structures in the Lieberkühn glands, goblet cells depletion, and severe leukocyte infiltration to the lamina propria (H&E × 40); D: colon tissues isolated from Sorafenib-treated DMH mice where regeneration of the goblet cells, the epithelial linings, restoration of the normal colon architecture, and reduction in the size of colon adenomas were noticed (H&E × 40)
Effect of Sorafenib on Wnt-pathway related gene expression
Effect of Sorafenib treatment on the expression of Wnt5a: As shown in Figure 4A, the expression of Wnt5a significantly decreased in DMH mice (Group B) and DMSO mice (Group C) by 2.57 and 5 folds (p<0.001 and p<0.0001) respectively, compared to normal control group A. Like group B, Sorafenib treatment (Group D) significantly decreased the expression of Wnt5a by 1.54-fold (p<0.001) compared to the normal group A. In addition, Sorafenib treatment (Group D) significantly attenuated the observed decrease in Wnt5a expression in Group C by 2.46 folds (p<0.0001).
Effect of Sorafenib treatment on the expression of GSK- 3β: DMH (group B) induced a significant decrease in GSK- 3β expression level by 7.57 folds (p<0.05) as shown in Figure 4B. Similarly, DMSO (Group C) induced a decrease in GSK- 3β expression level by 7.4 folds (p<0.05) compared to normal control group A. No significance difference was observed between groups B and C. As for Sorafenib treatment, it led to a significant increase in the expression of GSK-3β by 14 folds (p<0.001) compared to group B. Also, compared to the untreated DMSO control (Group C), Sorafenib treatment significantly increased the expression of GSK-3β by 13.83 folds (p<0.001).
Effect of Sorafenib treatment on the expression of APC: As shown in Figure 4C, the expression of APC significantly decreased in DMH mice (Group B) and DMSO mice (Group C) by 7.36 and 8.35 folds (p<0.0001) respectively, compared to normal control group A. on the other hand, Sorafenib treatment significantly increased the expression of APC by 12.53 folds (p<0.0001) compared to group B and by 13.52 folds compared to group C (p<0.0001).
Effect of Sorafenib treatment on the expression of β-Catenin: Figure 4D shows that DMH (Group B) was able to induce a significant increase in β-Catenin expression by 4.63 folds (p<0.0001) compared to the control (Group A) as shown in Figure 4D. DMSO (Group C) was also able to induce a significant increase in β-Catenin expression by 4.025 folds (p<0.0001) compared to group A. Treating DMH mice with Sorafenib induced an unexpected further significant increase in β-Catenin expression by 6.265 folds (p<0.0001) compared to Group C.
Effect of Sorafenib treatment on the expression of Cyclin D1: DMH (Group B) induced a significant increase in Cyclin D1 expression by 1 fold (p<0.05) compared to control (Group A) as shown in Figure 4E. DMSO (Group C) enhanced further the effect of DMH and induced further increase in Cyclin D1 expression by 5.02 folds (p<0.001) compared to normal control. However, Sorafenib treatment (Group D) induced a significant decrease in Cyclin D1 expression by 1.65 folds (p<0.05) compared to its control (Group C).
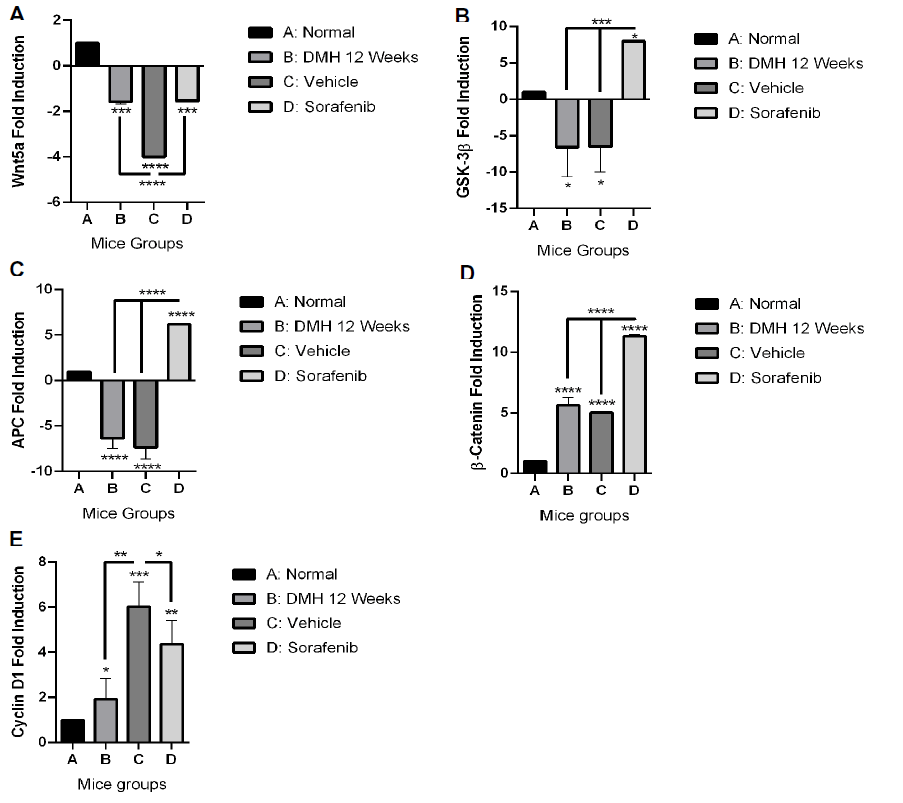
Figure 4: Effect of Sorafenib on the expression level of Wnt5a; (A): GSK3β; (B): APC; (C): β-catenin; (D): and Cyclin D1; (E): genes. Expression levels of treated and control groups were normalized to their respective GAPDH. Fold expression was determined relative to the control. All bars represent mean of three determinations ± SD. (*), (**), (***), and (****) on bars and on lines drawn upwards, that represent inter-categorical statistical significance, correspond to p<0.05, p<0.01, p<0.001, and p<0.0001, respectively
Since CRC is known as one of the common cancers worldwide with high mortality rates to date, and its predictable treatment methods are associated with a wide array of side effects [10], we aimed in our study to assess the antitumor potential of Sorafenib against a DMH-induced mouse model of CRC. We evaluated the proliferation through counting the number of polyps, performing a histopathological analysis, and assessing the mRNA expression level of Wnt signaling players, namely Wnt5a, GSK-3β, APC, β-Catenin and Cyclin D1 genes, as they aid to investigate in which step of colon carcinogenesis Sorafenib acts.
Colorectal carcinomas usually start as adenomatous polyps that are characterized by inflammation, neoplasia and hyperplasia [11]. Polyps are considered as beneficial intermediate biomarkers, which aid in studying the effects of drugs and compounds on chemically induced carcinogenesis [12]. In fact, the pro-carcinogen DMH is metabolized in the liver, then it reaches the colon where it produces the ultimate carcinogen methylazoxymethanol and Reactive Oxygen Species (ROS), which further alkylate DNA and initiate the development of colon carcinogenesis [13]. It has been shown that tumour induction by DMH produces adenoma-carcinoma sequence with histogenesis that matches the one existing in humans, and this is an advantage of DMH-induced animal models of CRC [14].
Our results show that Sorafenib treatment reduced the number of grown polyps in the colonic tissues of DMH-treated mice.
In addition, the conserved normal colonic architecture in Sorafenib treated group observed by the histopathological investigation further validated the effective chemopreventive effect of Sorafenib against colorectal polyps. These findings are consistent with a previous study by [15], where Sorafenib exerted an anti-tumor effect against mouse models of Hepatocellular Carcinoma (HCC).
Polyps in colonic tissues appear to emerge from genetic mutations, mostly in APC and β-catenin genes that are implicated in the Wnt signaling pathway [16]. In fact, most sporadic CRCs exhibit abnormal activation of the Wnt signaling pathway, which is considered the driver pathway of colorectal carcinogenesis [17]. Further, DMH is well-known to result in the aberrant activation of the Wnt signaling pathway [18]. In this context, our present study focused on assessing the Wnt signalling pathway at the level of the cell membrane (Wnt5a), the cytosol (APC, GSK3β and β-catenin) and the nucleus (Cyclin D1). Results show that this pathway was aberrantly activated in DMH-injected mice as evidenced by elevated gene expression levels of β-catenin and Cyclin D1 concomitant with down regulations in the tumour suppressor gene APC and GSK3β, compared to the control. These findings are consistent with previous results reported by [19], whereby the administration of DMH for a period of 12-weeks triggered the highest activation of the Wnt pathway compared to longer periods, indicating its activation at early stages of colorectal carcinogenesis. More specifically, our results did not show significant alterations in Wnt5a expression level indicating that DMH activated the Wnt signaling pathway through affecting its cytosolic and nuclear key players rather than its cellular membrane components. With respect to Sorafenib administration, results showed that this drug induced significant downregulations in the expression level of Cyclin D1 and β-catenin, as well as significant upregulations in the expression level of APC and GSK3β compared to the control. Interestingly, similar patterns were obtained upon comparing the effect of Sorafenib to that of the vehicle group (DMSO), implying that the observed anti-tumor effect is solely mediated by Sorafenib and not the vehicle. Our results are consistent with a previous study by [8] who found that Sorafenib suppresses the Wnt signaling in different cell lines in vitro and in a xenograft model of nude mice treated with Sorafenib which presented a reduced HepG2 tumor growth. Similarly, a recent study showed that Secretory Clusterin (sCLU) mediates GSK3β/β-catenin activation, thereby contributing to the malignant behavior and Cancer Stem Cell (CSC) phenotype of HCC cells upon Sorafenib treatment [4]. Another recent study showed that GSK-3β is overexpressed in HCC cells after Sorafenib treatment where the overexpression of GSK-3β confers HCC cell colony formation and xenograft tumor growth [20].
Our results pertaining to β-catenin expression are in concordance with a study done by [21] which showed that after 24 h of sorafenib treatment, the level of β-catenin was upregulated in SMMC-7721, MHCC-97H, and SK-Hep1 HCC cell lines; hence, these results suggest that the overexpression of β-catenin is closely correlated with sorafenib resistance in HCC. Another study by [22] demonstrated that HIF-2α was involved in sorafenib resistance via the regulation of β-catenin/c-Mycdependent pathways under hypoxic conditions in HCC. Further, it is important to note that our findings suggest that Sorafenib upregulated β-catenin expression at the transcriptional level only; however, they may not necessarily imply that the protein level of β-catenin is also elevated because various post-transcriptional and post-translational modifications can affect its protein level.
Compared to other treatments that affect CRC by various anti-proliferative mechanisms, our results concerning the effectiveness of the Sorafenib are similar to those revealed by [23] who reported an inhibitory effect of Taxifolin on Wnt signaling in DMH-induced mice. In their study, Taxifolin inhibited Wnt signaling by down-regulating the levels of regulatory metabolites such as TNF-α, COX-2, and Cyclin-D1. Other drugs that are empirically proven to exert their antitumor effects against CRC via inhibiting the Wnt signaling pathway include Vantictumab which binds to frizzled receptor, Tankyrase inhibitors that prevent β-catenin stabilization, and PORCN inhibitors that block Wnt secretion [24].
Finally, it is worthy to mention that the present findings are further corroborated by our previous research study where Sorafenib exerted selective cytotoxicity against colon cancer cells in HCT116 [9]. In that study, the antitumor activity of Sorafenib was shown to be independent of ROS level and p53 expression, thereby warranting further investigations on Sorafenib’s mechanism of action. Collectively, the present findings suggest that Sorafenib exerts its antitumor effects against CRC via targeting the Wnt signaling pathway at its transcriptional level.
Conclusion
In conclusion, the findings of our study suggest that Sorafenib effectively reduces colorectal tumour occurrence and progression in DMH-induced mouse model of CRC. The possible mechanisms proposed here show that the antitumor effect of Sorafenib is mediated through modulating the Wnt pathway which suppressed tumour cells proliferation and progression. The major limitation in this study is that our findings regarding Sorafenib’s ability to target the Wnt/β-catenin signaling pathway were merely based on mRNA levels. Therefore, further investigations at the protein level are needed in future studies.
Competing Interest
Authors have declared that no competing interests exist.
References
- Yuan S, Tao F, Zhang X, Zhang Y, Sun X, et al. Role of Wnt/β-catenin signaling in the chemoresistance modulation of colorectal cancer. BioMed Res Int. 2020:1-9.
- Recio-Boiles A, Cagir B. Colon Cancer. Treasure Island (FL): StatPearls. 2021.
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164.
- Zhang Y, Wang X. Targeting the Wnt/β‑catenin signaling pathway in cancer. J Hematol Oncol.2020;13:1-16.
- Zhang X, Wang L, Qu Y. Targeting the β-catenin signaling for cancer therapy. Pharmacol Res. 2020;160:104794.
- Kacan T, Nayir E, Altun A, Kilickap S, Babacan NA, et al. Antitumor activity of sorafenib on colorectal cancer. J Oncol Sci. 2016;2:53-57.
- Muche S, Kirschnick M, Schwarz M, Braeuning A. Synergistic effects of β-catenin inhibitors and sorafenib in hepatoma cells. Anticancer Res. 2014;34:4677-4683.
- Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;8:4997-5007.
- Al Hassan M, Reda F, Baltaji A, Usta J, Borjac B. Effect of Sorafenib on human colon cancer cells. BAU J Health Wellbeing. 2021;3:7.
- Siegel RL, Miller KD, Jemal A, Fuchs HE. Cancer statistics. CA Cancer J Clin.2020;71:7-33.
- Shussman N, Wexner SD. Colorectal polyps and polyposis syndromes. Gastroenterol Rep. 2014;2:1-15.
- Corpet DE, Taché S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer. 2002;43:1-21.
- Venkatachalam K, Vinayagam R, Arokia Vijaya Anand M, Isa NM, Ponnaiyan R. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: a review. Toxicol Res (Camb). 2020;9:2-18.
- Washington MK, Powell AE, Sullivan R, Sundberg JP, Wright N, et al. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterol. 2013;144:705-717.
- Kissel M, Berndt S, Fiebig L, Kling S, Ji Q, et al. Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget. 2017;8:107096.
- Sievers CK, Grady WM, Halberg RB, Pickhardt PJ. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev Gastroenterol Hepatol. 2017;11:723-729.
- Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol.2012;4:1-14.
- Kirsanov K, Fetisov T, Lesovaya EA, Maksimova V, Trukhanova L. Prevention of colorectal carcinogenesis by DNA-binding small-molecule curaxin CBL0137 involves suppression of Wnt signaling. Cancer Prev Res. 2015;13:53-64.
- El Joumaa MM, Taleb RI, Rizk S, Borjac JM. Protective effect of Matricaria chamomilla extract against 1, 2-dimethylhydrazine-induced colorectal cancer in mice. J Complement Integr Med. 2020;17:20190143.
- Zhang S, Gao W, Tang J, Zhang H, Zhou Y, Liu J, et al. The Roles of GSK-3β in regulation of retinoid signaling and sorafenib treatment response in hepatocellular carcinoma. Theranostics. 2020;10:1230-1244.
- Deng L, Sun J, Chen X, Liu L,Wu D. Nek2 augments sorafenib resistance by regulating the ubiquitination and localization of β-catenin in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:1-14.
- Liu F, Dong X, Lv H, Xiu P, Li T, et al. Targeting hypoxia-inducible factor-2α enhances sorafenib antitumor activity via β-catenin/C-Myc-dependent pathways in hepatocellular carcinoma. Oncol Let. 2015;10:778-784.
- Manigandan K, Manimaran D, Jayaraj RL, Elangovan N, Dhivya V, et al. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie. 2015;119:103-112.
- Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 2020;52:183-191.



