Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 9
Immunohistochemical expression of GATA3, CK5/6 and snail-1 in intrinsic subtypes of bladder carcinoma
Nehal K. Naga, Samir N. Mina, Ayman M. El-Saka, Safinaz H. El-Shorbagy and Alaa I. Amer*Alaa I. Amer, Department of Pathology, Faculty of Medicine, Tanta University, Tanta 31527, Egypt, Email: Alaa.amer@med.tanta.edu.eg
Received: 22-Jul-2023, Manuscript No. OAR-23-107711; Accepted: 01-Sep-2023, Pre QC No. OAR-23-107711 (PQ); Editor assigned: 27-Jul-2023, Pre QC No. OAR-23-107711 (PQ); Reviewed: 21-Aug-2023, QC No. OAR-23-107711 (Q); Revised: 28-Aug-2023, Manuscript No. OAR-23-107711 (R); Published: 04-Sep-2023
Abstract
Background: GATA3 binding protein has been described as a diagnostic immunomarker for urothelial carcinoma with high sensitivity and specificity. The purpose of this study was the evaluation of immunohistochemical based molecular classification of bladder cancer employing GATA3 and CK5/6, the relation between different subtypes and the available prognostic parameters of bladder cancer, correlation of the immunohistochemical expression of Snail-1 with various types of bladder carcinoma. Expression of GATA3, CK5/6 and Snail-1 immunohistochemistry was assessed in 80 formalin fixed embedded in paraffin blocks from selected cases of UBC (Trans Urethral Resection of bladder tumour 62 and Radical Cystectomy 18 specimens). Results: GATA3 showed positive nuclear expression in 75% of urothelial carcinoma cases, while all the studied cases of squamous cell carcinoma, adenocarcinoma and the neuroendocrine carcinoma small cell type were GATA3 negative. There was a significant relation between CK5/6 expression and high grade tumours as 100% of high scores were high grade with presence of muscle invasion. Moreover, by using CK5/6 and GATA3 cases were classified into three intrinsic molecular subtypes. Additionally, 62.5% of urothelial carcinoma cases showed positive nuclear and/or cytoplasmic Snail-1 expression, while most of studied cases of non-urothelial carcinoma (87.5%) were Snail-1 positive. Conclusions: GATA3 is considered as a specific marker for excluding nonurothelial tumours in primary urinary bladder carcinoma. GATA3 and CK5/6 could classify urothelial carcinoma cases into intrinsic molecular subtypes. Since, Snail-1 and CK5/6 expression was correlated significantly to high grade and muscle invasive tumours, we speculated that Snail-1 and CK5/6 considered as independent poor prognostic markers.
Keywords
immunohistochemistry, GATA3, CK5/6, snail-1, urinary bladder carcinoma (UBC)
Introduction
Urinary bladder carcinoma is one of the most common carcinomas all over the world. According to the recent cancer statistics 2020, it is the tenth most common cancer in men and the twelfth most common cancer in women in the America [1]. Bladder cancer is the fourth most common carcinoma in Egypt, accounting about 30% of all malignancies and accounting for the majority of urinary system malignancy [2].
Despite advances in intravesical treatment and surgical procedures, about 30% of non-muscle invasive bladder carcinoma cases progress to muscle invasion as well as up to 70% of cases have a high recurrence rate within the first year of diagnosis, therefore an appropriate prediction of progress is critical in the treatment of bladder cancer as the selection of biomarkers that can classify the high risk subgroups can improve the challenge [3].
Comprehensive messenger Ribonucleic Acid (mRNA) expression levels in the bladder carcinoma are recently used to classify urothelial carcinoma into different molecular subtypes. Luminal and basal intrinsic subtypes are the primary molecular subtypes, which resemble to the previously reported molecular subtypes of breast carcinoma [4]. Although additional research has expanded the molecular classification of urothelial carcinoma into 6 types, the basal and luminal subtypes continue to be the essential subtypes [5]. A meta-analysis study reported that immunohistochemical expression of GATA3 and Cytokeratin (CK) 5/6 can identify basal and luminal subtypes with accuracy more than 90% [4].
GATA3 protein binding is one of a family of tumour suppressor genes and it is essential for the growth, differentiation, proliferation, and apoptosis .It trans locates from the cytoplasm into the nucleus by importin-a to control gene expression. GATA3 affinity to importin-a regulation is mediated by Mitogen Activated Protein Kinase (MAPK), which enhances nuclear transport [6]. GATA3 is an immunomarker that is high expressed in breast and bladder cancers [7]. It is an accurate indicator of bladder cancer with only urothelial differentiation [8].
Cytokeratin’s belong to water insoluble intermediate filaments family, which present in the cytoplasm of epithelial cells and epithelial tumours, more than 25 subtypes of cytokeratin’s are recognized. Moreover, cytokeratin’s are divided into three categories: low-, high-, and pan keratin cocktail (AE1 and AE3), which retains a wide variety of cytokeratin’s [9]. CK5/6 is a reliable basal cell marker. Basal CK is detected in stratified epithelium basal cell layers and basal-like ductal epithelial cells of the breast [10]. CK5/6 usually present in squamous cell carcinoma in bladder that shows pure squamous differentiation with the absence of any urothelial component [11].
The Epithelial Mesenchymal Transition (EMT) is a procedure implicated in the invasion and migration of tumour cells. Epithelial cells differentiate through complex processes into highly invasive, motile mesenchymal-like cells that promote tumour metastasis. EMT is characterized by absence of E-cadherin expression and increase expression of many transcriptional suppressor of E-cadherin expression as Zeb, Slug, Twist, and Snail [12]. Snail is a family of zinc finger transcription factors that induces EMT by downregulating epithelial markers and upregulating mesenchymal markers. Thus, Snail could be linked to cancer development, metastasis, and treatment failure [13].
The aim of this work was to study the expression of GATA3 and CK5/6 to classify bladder carcinoma into luminal and basal subtypes, evaluation of the relation between luminal and basal subtypes and the available prognostic parameters of bladder cancer. Moreover, we aimed to study the correlation between Snail-1 expression and different subtypes of bladder carcinoma.
Materials and Methods
Study design
This retrospective study was carried out on 80 formalin fi xed embedded in paraffin blocks from selected cases of Urinary Bladder Carcinoma (UBC) specimens (62 Trans Urethral Resection of bladder tumour and 18 Radical Cystectomy specimens) from January 2018 to December 2021. The study was done after approval from the Research Ethics Committee (approval code number: 33820/5/20).
Data collection and histopathological evaluation
Clinical data were obtained from patient reports. Hematoxyline and Eosin (H and E) staining sections were reviewed for diagnosis and for evaluation of various histopathological features which include the pathologic grade, depth of invasion, lymph vascular invasion.
The UBC cases were microscopically classified according to th e World Health Organization (WHO) system into: Urothelial Carcinoma Cases High Grade (UCHG), Urothelial Carcinoma Low Grade (UCLG) tumours, squamous cell carcinoma, adenocarcinoma cases and neuroendocrine carcinoma [14]. Staging of the studied cases was done according to American Joint Committee on Cancer (AJCC) Tumour Node Metastasis (TNM) Staging of Urinary Bladder cancers (8th edition) [15].
Immunohistochemical staining
Bladder cancer sections (5 um) were deparaffinised then hydrated, and immersed in 3% hydrogen peroxide for 20 minutes for blocking endogenous peroxidase activity at room temperature then washed by Phosphate Buffer Solution (PBS) for few minutes and left for drying.
GATA3 a mouse monoclonal antibody (Cat. No. Sc-268, 1:100 dilution Santa Cruz Biotechnology, Inc), CK5/6 a mouse monoclonal antibody (Cat. No. Sc-53262, 1:100 dilution Santa Cruz Biotechnology, Inc) and Snail-1 a rabbit polyclonal antibody (Cat. No. A5243, 1: 50 dilution AB clonal Biotechnology) were placed on each slide for 30 minutes. Two to three drops of the secondary biotinylated antibody were placed on the slides for 45 minutes then the slides were dried. The slides were incubated for 5-10 minutes in Diamino-Benzedine (DAB) chromogen then washed in tap water, counter stain with Mayer’s hematoxylin for one minute was done, and then washed in tap water, and sections were dehydrated in ascending grades of alcohol, cleared in xylene, and then covered by using Canada balsam [16]. Negative controls were acquired by skipping the step of primary antibody incubation
Interpretation of GATA3, CK5/6 and Snail-1 positivity: Using the semi quantitative score (immunoreactive score), that incorporates both: the percentage of immune reactive cells and the intensity of staining. The percentage of immunoreactive cells: 0% (0), 1%-49% (+1), 50%-70% (+2), >70% (+3). The intensity of staining was scored as the following: negative (0), weak staining (+1), medium staining (+2), strong staining (+3). An IHC result was assigned by using semi quantitative scoring system.
Immunoreactive score was calculated by multiplying the percentage of immunoreactive cells by the intensity of staining: (0): Negative (0), (1-3): Weakly positive (+1), (4-6): Moderately positive (+2), >6: Strongly positive (+3). For statistical purpose, Snail-1 immunohistochemical scores (0 and +1) were considered negative and immunohistochemical scores of (+2 and +3) were considered positive [17-19].
Statistical analysis
The collected data was statistically analysed using Statistical Package for the Social Sciences (SPSS) software statistical computer version 20. Data were expressed in terms of frequencies (number of cases) and percentages for categorical variables and range, mean ± Standard Deviation (SD) for continuous variables. Chi-square test was used for categorical variables, to compare different groups. Fishers Exact or Monte Carlo correction was used to correct chi-square when more than 20% of the cells have expected count less than 5. The level of significance was selected at p-value <0.05.
Results
Clinicopathologic characteristics of urinary bladder carcinoma cases
The eighty studied cases were divided into 64 cases of urothelial carcinoma (80%) and non-urothelial carcinoma (20%), including 10 cases of SCC (12.5%), 5 cases of adenocarcinoma (6.3%) and 1 case of neuroendocrine small cell carcinoma (1.3%). The clinicopathological features of the studied cases of urinary bladder carcinoma are summarized in Table 1. The age of the studied cases ranged between 47 and 86 years, with a mean age 64.69. Male to female ratio was 9:1. Urothelial carcinoma cases were categorized into low grade 14 cases and high grade 50 cases out of 64 cases urothelial carcinomas. All the studied cases of Squamous cell carcinoma, adenocarcinoma and the neuroendocrine carcinoma small cell type were of high histological grade. Most of the studied cases showed muscle invasion (T2a) at least 56 cases (70%). Vascular invasion was detected in 13 cases (16.2%)
Tab. 1. Clinicopathological features of studied cases of urinary bladder carcinoma
| Clinicopathological features | (80) Total | % |
|---|---|---|
| Age (Mean ± SD.) | 64.36 ± 8.26 | |
| Gender | ||
| Male | 72 | 90 |
| Female | 8 | 10 |
| Histopathological type | ||
| Urothelial | 64 | 80 |
| Non urothelial | 16 | 20 |
| SCC | 10 | 12.5 |
| Adenocarcinoma | 5 | 6.25 |
| Neuroendocrine carcinoma | 1 | 1.25 |
| Grade | ||
| High grade | 66 | 82.5 |
| Low grade | 14 | 17.5 |
| Degree of muscle invasion | ||
| Non muscle invasion | 24 | 30 |
| Muscle invasion | 56 | 70 |
| Vascular invasion | ||
| Absent | 67 | 83.8 |
| Present | 13 | 16.2 |
GATA3, CK5/6 and snail-1 immunohistochemical results
Forty-eight cases showed positive nuclear GATA3 expression (60%) (21 cases showed moderate positivity +2 and 27 cases were strong GATA3 positive +3).The remaining 32 cases were GATA3 negative (Figure 1). Fifty-six c ases s howed p ositive C K5/6 expression (70%) (23 cases showed CK5/6 positivity +1, 23 cases showed positivity +2 and 10 cases were positive +3), while 24 cases were CK5/6 negative (30%) (Figure 2). Out of our 80 cases, 54cases showed Snail-1 expression (67.5%) distributed as 26 cases showed Snail-1 score +2 and 28 cases were Snail-1score +3), while 26 cases(32.5%) were negative distributed as 7 cases of Snail1showed no stain and 19 cases showed Snail-1 score +1. (Figure 3), Table 2.
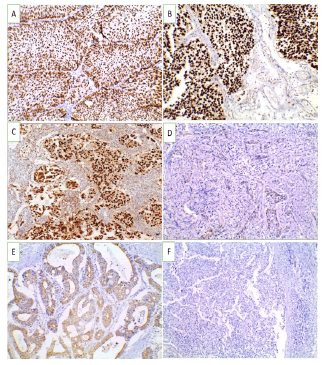
Figure 1: GATA3 immunoexpression in studied UBC cases (X 200) (A). Non-invasive low grade urothelial carcinoma showing positive nuclear expression (+3) (B). Non-invasive high grade urothelial carcinoma showing positive nuclear expression (+3) (C). Invasive urothelial carcinoma showing positive nuclear expression (+3) (D). Squamous cell carcinoma showing negative expression of GATA3 (E). Adenocarcinoma showing negative nuclear expression of GATA3 (F). Neuroendocrine carcinoma showing negative expression of GATA3
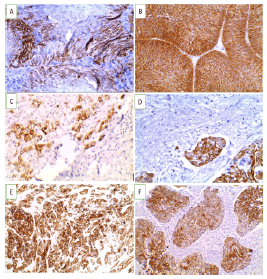
Figure 2: CK5/6 immunoexpression in studied UBC cases (A-D X 400) (A). Non-invasive high grade urothelial carcinoma showing positive cytoplasmic and membranous expression (+2) (B). Non-invasive high grade urothelial carcinoma showing positive cytoplasmic and membranous expression (+3) (C). Invasive high grade urothelial carcinoma showing positive cytoplasmic and membranous expression (+1) (D). Invasive urothelial carcinoma with vascular embolus showing positive cytoplasmic and membranous expression (+3).(E-FX200) (E). Invasive high grade urothelial carcinoma with squamous differentiation showing positive cytoplasmic and membranous expression (+3) (F). Squamous cell carcinoma showing positive cytoplasmic and membranous expression (+3)
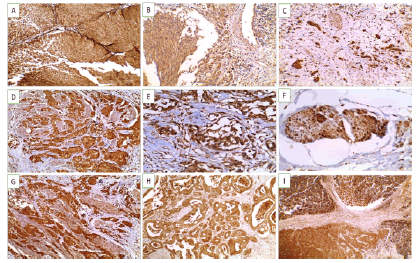
Figure 3: Snail-1 immunoexpression in studied UBC cases(A-D X 200) (A). Non-invasive high grade urothelial carcinoma showing positive cytoplasmic and nuclear expression (+3) (B). Invasive laminal low grade urothelial carcinoma showing mainly cytoplasmic expression (+2) (C). Invasive high grade urothelial carcinoma showing positive cytoplasmic and nuclear expression (+3) (D). Invasive high grade urothelial carcinoma infiltrate muscularis propria showing positive cytoplasmic and nuclear expression (+3) .(E-FX400) (E). Lipoid cell variant showing positive cytoplasmic and nuclear expression (+3) (F). Invasive urothelial carcinoma with vascular emboli showing cytoplasmic and nuclear expression (+3) (G-I X200) (G). Squamous cell carcinoma showing positive cytoplasmic and nuclear expression (+3) (H). Adenocarcinoma showing positive expression of (+2) (I). Neuroendocrine carcinoma showing positive nuclear expression (+3).
Tab. 2. Distribution of the studied cases according to GATA3, CK 5/6 expression and Snail-1 expression (n=80)
| Total | Histologic type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Urothelial carcinoma | Non urothelial carcinoma | ||||||||
| Non-invasive | Invasive | Squamous cell carcinoma | Adenocarcinoma | Neuroendocrine carcinoma | |||||
| GATA3 | |||||||||
| Negative |
32(40%) |
0 | 16(29.6%) | 10(100%) | 5(100%) | 1(100.0%) | |||
|
Positive |
+2 |
21(26.2%) | 0 | 21(38.9%) | 0 | 0 | 0 | ||
|
+3 |
27(33.8%) | 10(100%) | 17(31.5%) | 0 | 0 | 0 | |||
| CK 5/6 | |||||||||
| Negative | 24 (30%) | 6 (7.5%) | 12 (15%) | 0 | 5 (6.25%) | 1 (1.25%) | |||
|
Positive |
+1 | 23(28.75%) | 2 (2.5%) | 20 (25%) | 1 (1.25%) | 0 | 0 | ||
| +2 | 23(28.75%) | 1 (1.25%) | 19(23.75%) | 3 (3.75%) | 0 | 0 | |||
| +3 | 10 (12.5%) | 1 (1.25%) | 3 (3.75%) | 6 (7.5%) | 0 | 0 | |||
| Snail-1 | |||||||||
| Negative | 0 | 7 (8.75%) | 4 (5%) | 3 (3.75%) | 0 | 0 | 0 | ||
| +1 | 19 (23.75%) | 5 (6.25%) | 12 (15%) | 1 (1.25%) | 1 (1.25%) | 0 | |||
| Positive | +2 | 26 (32.5%) | 2 (2.5%) | 16 (20%) | 4 (5%) | 4 (5%) | 0 | ||
| +3 | 28 (35%) | 3 (3.75%) | 19 (23.75%) | 5 (6.25%) | 0 | 1 (1.25%) | |||
By using CK5/6 and GATA3 immunomarkers in the current study, three intrinsic molecular subtypes of urothelial carcinomas were stratified as luminal (CK5/6-ve/GATA3+ve) composed of 18 cases (16 cases of pure urothelial carcinoma and 2 cases with divergent differentiation), basal (CK5/6+ve/GATA3-ve) composed of 16 cases (15 cases of pure urothelial carcinoma and one case with divergent differentiation) and mixed (CK5/6+ve/ GATA3+ve) composed of 30 cases (16 cases of pure urothelial carcinoma and 14 cases with divergent differentiation). These three molecular classifications was significantly correlated with the histologic types, grade, degree of muscle invasion P-value was ≤ 0.05 as demonstrated in Table 3.
Tab. 3. Relation between molecular subtypes, histologic types, grade and muscle invasion in urothelial carcinoma cases (n= 64).
| CK5/6 / GATA3 | X2 | MCp | ||||
|---|---|---|---|---|---|---|
| Histologic type | Basal ±) (n= 16) |
Luminal ( ) )(n= 18) |
Mixed (+/+) (n= 30) |
|||
| Pure urothelial | 15 (93.7%) | 16 (88.9%) | 16 (53.3%) | 11.212 | 0.004 | |
| With differentiations | 1 (6.3%) | 2 (11.1%) | 14 (46.7%) | - | - | |
| Grade | Low grade | 0 | 9 (50%) | 5 (16.7%) | 12.633 | 0.001 |
| High grade | 16 (100%) | 9 (50%) | 25 (83.3%) | |||
| Degree of muscle invasion |
Non muscle invasive |
0 | 10 (55.6%) | 12 (40%) | 12.381 | 0.002 |
|
Muscle invasive (pT2 at least) |
16 (100%) | 8 (44.4%) | 18 (60%) | |||
Snail-1 expression is insignificantly correlated with histologic type, vascular invasion and GATA3 expression (p-value was >0.05). On the other hand, it was significantly correlated with the tumour grade, degree of muscle invasion and CK5/6 expression (p-value was <0.05) of studied urinary bladder carcinoma cases as in Table 4.
Tab. 4. Relation between Snail-1 with different clinicopathologic parameters, CK 5/6 and GATA3 expression (n=80).
| Histologic type | Snail-1 | X2 | MCp | |||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||
| No stain (n= 7) |
+1 (n= 19) |
+2 (n= 26) |
+3 (n= 28) |
|||||
| Urothelial carcinoma | 7 (100%) | 17 (89.5%) | 18 (69.2%) | 22 (78.6%) | 9.809 | 0.287 | ||
| Non urothelial carcinoma | 0 | 2 (10.5%) | 8 (30.8%) | 6 (21.4%) | ||||
| Grade | High | 5 (71.4%) | 9 (47.4%) | 24 (92.3%) | 28 (100%) | 20.282 | <0.001 | |
| Low | 2 (28.6%) | 10 (58.8%) | 2 (11.1%) | 0 | ||||
| Degree of muscle invasion | Non muscle invasive | 2(28.6%) | 10(52.6%) | 4(15.4%) | 8(28.6%) | 9.996 | 0.018 | |
| Muscle invasive | 5(71.4%) | 9(47.4%) | 22(84.6%) | 20(71.4%) | ||||
| Vascular invasion | No | 7 (100%) | 18 (94.7%) | 23 (88.5%) | 19 (67.9%) | 7.028 | 0.054 | |
| Yes | 0 | 1 (5.3%) | 3 (11.5%) | 9 (32.1%) | ||||
| CK 5/6 | ||||||||
| Negative | 4 (57.1 %) | 7 (36.9%) | 10 (38.5 %) | 3 (10.7%) | 25.459 | 0.001 | ||
| +1 | 3 (42.9%) | 10 (52.6%) | 3 (11.5%) | 7 (25%) | ||||
| +2 | 0 | 2(10.5%) | 10 (38.5%) | 11 (39.3%) | ||||
| +3 | 0 | 0 | 3 (11.5%) | 7 (25%) | ||||
| GATA3 | ||||||||
| Negative | 2 (28.6%) | 6 (31.6%) | 13 (50%) | 11 (39.3%) | 3.722 | 0.733 | ||
| +2 | 2 (28.6%) | 4 (21%) | 7 (26.9%) | 8 (28.6%) | ||||
| +3 | 3 (42.8%) | 9 (47.4%) | 6 (23.1%) | 9 (32.1%) | ||||
Discussion
Urinary bladder carcinoma is an international health problem constituted an essential cause of morbidity and mortality. It is the 10th most common carcinoma in the world and one of the most common carcinoma in men [1].
As urinary bladder carcinoma has markedly various behavioural features, patients with the same disease stage may have different clinical course and outcomes following the same treatment strategy [20]. This together with the high likelihood of recurrence, have made the development of new urinary markers with the hope of appropriate diagnosis, grading, with prediction of the therapeutic response of the tumours [21].
In the current study, we assessed the expression of GATA3, CK5/6 and Snail-1 in various types of urinary bladder carcinoma and the assessment of the role of GATA3 and CK5/6 in the molecular classification of bladder cancer into luminal and basal subtypes.
GATA3 described as a biomarker that is highly expressed in bladder carcinomas, with high sensitivity especially in pure urothelial differentiation [22]. In addition, absence of GATA3 expression was linked with higher grade tumours, and poorer survival outcomes in most previous studies [4].
In the current study, 48 cases out of 64 studied UC cases showed positive nuclear GATA3 expression, while all the studied cases of squamous cell carcinoma, adenocarcinoma and the neuroendocrine carcinoma small cell type were GATA3 negative. Among those UC cases, strong nuclear GATA3 expression (+3) was detected in most of LGUC, while regarding HG infiltrating UC, 75% of cases showed positive nuclear GATA3 expression, while only 25% were GATA3 negative. This finding agreed with Chang et al [23-25]. Who reported that positive staining for GATA3 was detected in 80%, 70% and 80% of their studied UC cases, respectively? These percentages were slightly lower than those obtained by Gulmann et al [26-28]. Who observed 93%, 90% and 88% overall GATA3 positivity in UC cases, respectively. All included cases of SCC in the present study were GATA3 negative. These findings matched with those of Gulmann et al, who found no GATA3 expression in pure SCC of the bladder [26]. In contrast reported that an overall 7% and 5% of their studied cases of well-differentiated SCCs were positive for GATA3, respectively [24, 29]. They concluded that GATA3 can be used to differentiate urothelial carcinoma with squamous differentiation from SCC. All included adenocarcinoma cases in this study were GATA3 negative. This result agreed with Rao et al [8]. Who stated that all their adenocarcinoma cases showed GATA3 negative expression and concluded that GATA3 may be a useful marker in differentiating UC with glandular differentiation from primary adenocarcinoma [30].
The only case of neuroendocrine small cell carcinoma in this study was GATA3 negative. This agreed with Wang et al. who stated that bladder neuroendocrine small cell carcinoma showed negative GATA3 expression. Verduin et al found that only 1 of their 6 cases (17%) of small cell neuroendocrine carcinomas exhibited GATA3 labelling that tumour with mixed components of UC showed more positivity in the non-small cell components [22]. Our results were in contrast with a study made by Mahmood et al. who found that bladder neuroendocrine small cell carcinoma showed positivity for GATA3 [31].
Cytokeratin 5/6 is a cytokeratin expressed in a squamous epithelial cell. It is used generally as a marker of squamous differentiation,that indicates the basal subtype. CK5/6 expression in urothelial carcinoma was associated with poor outcomes in various studies [11]. This study demonstrated CK5/6 expression in 70 % of all studied cases and 71% of the urothelial carcinoma cases. These results agreed with previous studies [32]. In contrast, other studies documented presence of CK5/6 expression in urothelial carcinoma cases which ranged from 19% to 57% only [33].
In the current study, CK5/6 and GATA3 categorized cases of urothelial carcinoma into three intrinsic molecular subtypes. Both markers are used widely in routine investigation and have an inverse expression pattern. Depending on immunoreactivity for GATA3 and CK5/6, cases are classified into 3 groups: 16 cases were GATA3 -ve/ CK5/6 +ve (basal), 18 cases were GATA3 +ve/ CK5/6 -ve (luminal) and 30 cases were GATA3 +ve / CK5/6 +ve. The latter group was accompanied by intermediated prognostic features between basal and luminal types.
There was significant association between stratified int rinsic molecular subtypes, grade and muscle invasion as basal subtype (CK5/6+ve/GATA3-ve) showed high incidence with poor prognostic factors as high grade, presence of muscle invasion. These results agreed with Calvete et al., who explained that basal bladder tumours which express Cancer Stem Cell (CSC) markers characteristic of epithelial mesenchymal transition, as cytokeratins 5, 6, and 14 [34, 35].
Snail-1 shows a wide spectrum of many biological functions. Additionally, the regulation of cell movements, proliferation and survival, immune suppression and generation of stem cell properties. However, there are more processes for Snail-1- regulation to be discovered [36].
Among UC cases, all strong nuclear and cytoplasmic Snail-1 expression (+3) was detected in HGUC. This finding agreed with who stated that the Snail-1 expression was significant associated with a disagreed with the current study in which their study showed Snail-1 reactions superior in low grade and early-stage carcinomas, this controversy maybe related to using different clone type and dilution [37, 38]. All included cases of SCC in the current study showed Snail-1 positivity. Other studies showed somewhat different result regarding that Snail-1 was highly significant in 60% of the SCC cases [39]. Most of HGUC cases showed Snail-1 positivity. Out of 50 the high-grade cases, 22 cases showed positive Snail-1 expression (+3) and 16 cases showed positive Snail-1 expression (score +2) while 12 were negative (as 0 or +1). The correlation between Snail-1 expression and the pathological grade had statistical significance. Th is ag reed wi th the results obtained by Kosaka et al. [37].
Regarding the vascular invasion in bladder carcinoma cases, 12 cases out of 13 cases showed Snail-1 positivity Also indicated that Snail-1 expression was close related to lympho-vascular relation. In contrast to this study found that there are no significant associations were observed between Snail-1 expression and the pathological stage, grade, and vascular invasion [37, 40].
In the current study, regarding correlation between Snail-1, CK5/6 and GATA3, there was significant direct correlation between CK5/6 and Snail-1 expression. However, no significant correlation was found between Snail-1 expression and GATA3. It is recommended that molecular subtyping of bladder carcinoma may be a successful tool in determination patient's response to different types of treatments and their outcomes, IHC based molecular subtyping, with clinical and pathological parameters increase their efficacy which seems to be more applicable than expensive tissue consuming mRNA based tumour profiling, further study of the significance of Snail-1 as a prognostic marker, its involvement in the control of EMT and metastasis and its role in drug resistance is recommended.
Conclusion
GATA3 is positive only in urothelial carcinoma cases while all cases of SCC and adenocarcinoma lack expression. So, GATA3 is a specific marker for excluding non-urothelial tumors. There was significant co-relation between Snail-1 and CK5/6 as both are increased in expression with high grade and muscle invasive tumors, considered as independent prognostic markers, may improve the prediction of bad prognosis for patients with bladder carcinoma.
Conflicts of Interest
The authors have no conflict of interest to declare
Funding
The authors did not receive support from any organization for the submitted work.
Availability Of Data And Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
Not applicable
References
- Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, et al. Epidemiology of bladder cancer. Medical sciences. 2020 Mar 13;8:15.
- Mohamed DO, Sayed MM, Abdelkawi IF, Elshoieby MH, Khallaf SM, et al. Bladder preservation versus radical cystectomy in transitional cell carcinoma and squamous cell carcinoma muscle invasive bladder cancer. Curr Urol. 2021;15:11.
- Boegemann M, Krabbe LM. Prognostic implications of immunohistochemical biomarkers in non-muscle-invasive blad cancer and muscle-invasive bladder cancer. Mini Rev Med Chem. 2020;20:1133-1152.
- Bejrananda T, Kanjanapradit K, Saetang J, Sangkhathat S. Impact of immunohistochemistry-based subtyping of GATA3, CK20, CK5/6, and CK14 expression on survival after radical cystectomy for muscle-invasive bladder cancer. Sci Rep. 2021;11:21186.
- Makboul R, Hassan HM, Refaiy A, Abdelkawi IF, Shahat AA, et al. A simple immunohistochemical panel could predict and correlate to clinicopathologic and molecular subgroups of urinary bladder urothelial carcinoma. Clin Genitourin Cancer. 2019;17:712-719.
- Heabah NA, Aref MF, Mokhtar MA. Comparative Study of GATA3 and CD147 Expression in Urinary Bladder Carcinoma. Nat Sci. 2019; 17;100-111.
- Miettinen M, Cue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, et al. GATA 3â??a multispecific but potentially useful marker in surgical pathologyâ??a systematic analysis of 2500 epithelial and non-epithelial tumors. Am j surg pathol. 2014;38:13.
- Rao Q, Williamson SR, Lopez-Beltran A, Montironi R, Huang W, et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: extended immunohistochemical profiles emphasizing novel markers. Mod Pathol. 2013;26:725-732.
- Akhtar M, Rashid S, Gashir MB, Taha NM, Al Bozom I. CK20 and CK5/6 immunohistochemical staining of urothelial neoplasms: A perspective. Advances in Urology. 2020.
- Hemida AS, Aiad HA, Hassan NA, Al Sharaky DR. Fibroblast activation protein (FAP) expression in CK5/6 expressed (Basal subtype) and CK20 expressed (Luminal subtype) urothelial bladder carcinoma: an immunohistochemical study. J Immunoass Immunochem. 2022;43:618-633.
- Hashmi AA, Hussain ZF, Irfan M, Edhi MM, Kanwal S, et al. Cytokeratin 5/6 expression in bladder cancer: association with clinicopathologic parameters and prognosis. BMC res notes. 2018;11:1-5.
- Singh R, Mandhani A, Agrawal V, Garg M. Immunohistochemical studies to examine the diagnostic and prognostic implications of epithelial-to-mesenchymal transition in patients with urothelial carcinoma of the bladder. SN Compr Clin Med. 2019;1:277-286.
- Roth B, Jayaratna I, Sundi D, Cheng T, Melquist J, et al. Employing an orthotopic model to study the role of epithelial-mesenchymal transition in bladder cancer metastasis. Oncotarget. 2017;8:34205.
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organsâ??part A: renal, penile, and testicular tumours. Eur urol. 2016;70:93-105.
- Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, et al. AJCC cancer staging manual. N Y: springer; 2017.
[Google Scholar] [CrossRef]
- Buchwalow IB, Böcker W, Buchwalow IB, Böcker W. Immunostaining enhancement. mmunohistochemistry: basics methods. 2010:47-59.
- Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin cancer res. 2007;13:4769-4776.
- Miyamoto H, Izumi K, Yao JL, Li Y, Yang Q, et al. GATA binding protein 3 is down-regulated in bladder cancer yet strong expression is an independent predictor of poor prognosis in invasive tumor. Hum pathol. 2012;43:2033-2040.
- Sikic D, Keck B, Wach S, Taubert H, Wullich B, et al. Immunohistochemiocal subtyping using CK20 and CK5 can identify urothelial carcinomas of the upper urinary tract with a poor prognosis. PLoS One. 2017;12.
- Als AB, Dyrskjøt L, von der Maase H, Koed K, Mansilla F, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407-4414.
- Miremami J, Kyprianou N. The promise of novel molecular markers in bladder cancer. Int j mol sci. 2014;15:23897-23908.
- Verduin L, Mentrikoski MJ, Heitz CT, Wick MR. The utility of GATA3 in the diagnosis of urothelial carcinomas with variant morphologic patterns. Appl Immunohistochem Mol Morphol. 2016;24:509-513.
- Chang A, Amin A, Gabrielson E, Illei P, Roden RB, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am j surg pathol. 2012;36:1472.
- Liang Y, Heitzman J, Kamat AM, Dinney CP, Czerniak B, et al. Differential expression of GATA-3 in urothelial carcinoma variants. Hum pathol. 2014;45:1466-1472.
- Hayashi T, Sentani K, Goto K, Oo HZ, Abdi H, et al. Mp78-03 Immunohistochemical Classification Using Urothelial Differentiation Markers Can Stratify Prognosis In Patients With Muscle Invasive Bladder Cancer. J Urol. 2018;199.
- Gulmann C, Paner GP, Parakh RS, Hansel DE, Shen SS, et al. Immunohistochemical profile to distinguish urothelial from squamous differentiation in carcinomas of urothelial tract. Hum pathol. 2013;44:164-172.
- Zhao L, Antic T, Witten D, Paner GP, Taxy JB, et al. Is GATA3 expression maintained in regional metastases?: a study of paired primary and metastatic urothelial carcinomas. The American Journal of Surgical Pathology. 2013;37:1876-1881.
- Paner GP, Annaiah C, Gulmann C, Rao P, Ro JY, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Human Pathology. 2014;45:1473-1482.
- Helmy NA, Khalil HK, Kamel NN, AboelFadl DM. Role of GATA3, CK7, CK20 and CK14 in distinguishing urinary bladder squamous cell carcinoma and urothelial carcinoma with squamous differentiation. Egyptian Journal of Pathology. 2015;35:133-138.
- Kashyap A, Shukla S. Adenocarcinoma of urinary bladder in a 55-year-old female: An unusual case report. Indian Journal of Case Reports. 2017:200-202.
[Google Scholar] [CrossRef]
- Mahmood H, Fatima H, Faheem M. Management of Small Cell Neuroendocrine Carcinoma of Urinary Bladder, A Case Series from Northern Pakistan. Cancer Therapy and Oncology International Journal. 2018;9:13-16.
[Google Scholar] [CrossRef]
- Sjödahl G, Lövgren K, Lauss M, Patschan O, Gudjonsson S, et al. Toward a molecular pathologic classification of urothelial carcinoma. The American journal of pathology. 2013;183:681-691.
- Jangir H, Nambirajan A, Seth A, Sahoo RK, Dinda AK, et al. Prognostic stratification of muscle invasive urothelial carcinomas using limited immunohistochemical panel of Gata3 and cytokeratins 5/6, 14 and 20. Annals of diagnostic pathology. 2019;43:151397.
[Google Scholar] [CrossRef]
- Calvete J, Larrinaga G, Errarte P, Martin AM, Dotor A, et al. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Human pathology. 2019;91:61-68.
- Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nature Reviews Urology. 2014;11:400-410.
- Wu Y, Zhou BP. Snail: more than EMT. Cell adhesion and migration. 2010;4:199-203.
- Kosaka T, Kikuchi E, Mikami S, Miyajima A, Shirotake S, et al. Expression of snail in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clinical Cancer Research. 2010;16:5814-5823.
- Stepan AE, Ciurea RN, DrÄ?goescu PO, Florescu MM, Stepan MD. Immunoexpression of transcription factors in urothelial bladder carcinomas. Rom J Morphol Embryol. 2017;58:863-869.
- Khalil H, Hammam OA, Kamel A. Detection of Epithelial-Mesenchymal Transition Markers in High Grade Bladder Cancer and Special Variants of Urothelial Carcinoma. Asian Pacific Journal of Cancer Prevention: APJCP. 2022;23:2079.
- Nomura S, Suzuki Y, Takahashi R, Terasaki M, Kimata R, et al. Snail expression and outcome in T1 high-grade and T2 bladder cancer: a retrospective immunohistochemical analysis. BMC urology. 2013;13:1-6.