Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 6
Experimental measurement of entrance skin dose in IMRT and VMAT for cervical cancer in the pelvic region using a female phantom and optically stimulated luminescence dosimeters
Ahlam Azalmad1*, Abour Mohamed2 and Mohamed Hilal12LSIB Laboratory, Faculty of Sciences and Technologies, Hassan II University of Casablanca, Mohammedia, Morocco
Ahlam Azalmad, The Health Sciences and Technologies Laboratory, Higher Institute of Health Sciences, University Hassan 1st, Settat, Morocco, Email: a.azalmad@uhp.ac.ma
Received: 17-Jul-2025, Manuscript No. OAR-25-170497; , Pre QC No. OAR-25-170497 (PQ); Editor assigned: 22-Jul-2025, Pre QC No. OAR-25-170497 (PQ); Reviewed: 05-Aug-2025, QC No. OAR-25-170497; Revised: 13-Aug-2025, Manuscript No. OAR-25-170497 (R); Published: 15-Sep-2025
Abstract
Purpose: Minimizing radiation-induced skin toxicity while ensuring optimal tumor coverage is a critical challenge in cervical cancer radiotherapy. This study compares Entrance Skin Dose (ESD) and target coverage for Intensity-Modulated Radiation Therapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT), highlighting their implications for radiation protection.
Materials and methods: Eight Optically Stimulated Luminescence (OSL) dosimeters, providing precise point measurements of ESD with high spatial resolution, were placed at clinically relevant anatomical sites on a female anthropomorphic phantom. The phantom underwent a planning CT scan, and IMRT and VMAT plans were simulated in Eclipse using 6 MV photon beams on a Varian TrueBeam linear accelerator.
Results: IMRT delivered slightly higher skin doses (0.589 Gy measured vs. 0.566 Gy calculated) compared to VMAT (0.494 Gy measured vs. 0.477 Gy calculated), with an average relative increase of ~0.5%.
Conclusion: VMAT demonstrates lower skin exposure and may be preferable for patients at higher risk of radiation-induced skin toxicity. OSL dosimetry allowed accurate ESD assessment, supporting radiation protection by confirming doses remain within safe limits and showing strong agreement with ICRU Reports 50 and 83.
Keywords
Cervical cancer; IMRT; VMAT; Entrance skin dose; OSL dosimetry; Radiation protection; Anthropomorphic phantom; Skin toxicity
Introduction
Cervical cancer continues to be one of the most prevalent cancers among women globally, especially in low and middle-income nations, where access to early detection and treatment is restricted [1]. Radiotherapy plays a central role in the management of cervical cancer, especially for locally advanced stages, either as a standalone treatment or in combination with chemotherapy [2].
Advancements in external beam radiotherapy have significantly improved dose conformity and sparing of healthy tissues. T hese conformal techniques allow for better tumor targeting while decreasing exposure to surrounding tissue, making them increasingly preferred in clinical practice [3].
However, the precision of these advanced techniques depends heavily on accurate patient setup and reproducibility. Setup uncertainties organ motion and anatomical changes can affect the actual dose delivered, potentially compromising treatment efficacy or increasing unintended exposure to healthy tissues. Therefore, verifying the consistency between planned and delivered doses is essential for ensuring treatment accuracy and safety [4].
One critical aspect of radiation protection in radiotherapy is the assessment of the Entrance Skin Dose (ESD) the radiation dose received at the patient’s skin surface where the beam enters. Excessive ESD can cause acute skin reactions such as erythema or desquamation and must be monitored closely to comply with safety guidelines and the ALARA (As Low as Reasonably Achievable) principle [5]. Optically Stimulated Luminescence (OSL) dosimeters, such as Landauer nanoDots, provide a sensitive, non-invasive and reliable method for in vivo ESD measurement without disrupting treatment setup [6,7].
T his study aims to quantify the entrance skin dose in cervical cancer patients treating by IMRT and VMAT, using OSL nanoDot dosimeters and to compare these measurements with values calculated by the Treatment Planning System (TPS), ensuring compliance with international dosimetric and radiation protection standards [8].
Materials and Methods
To evaluate entrance skin doses in cervical cancer radiotherapy, a female anthropomorphic phantom was used. This approach allowed accurate measurement of skin doses at multiple anatomical sites without exposing patients to additional radiation. The phantom provides stable and reproducible anatomy, enabling precise and repeatable dosimetry as well as systematic comparison of IMRT and VMAT treatment plans under controlled conditions, in full compliance with radiation protection principles.
Phantom description
T he Female ATOM® phantom (Model 702, CIRS Inc., Norfolk, VA, USA) was used to simulate female pelvic anatomy (Figure 1).
Composed of 25 mm-thick axial slices, it includes anatomically accurate structures such as the uterus, bladder, rectum, ovaries and pelvic bones. The phantom allows insertion of tissue-equivalent dosimetry plugs, including holders for 1 × 1 cm OSLDs, enabling precise in-phantom dose measurements. Its realistic tissue hetero geneity supports accurate validation of Treatment Planning System (TPS) dose calculations in gynecological radiotherapy [9].
Fig. 1. Female phantom.
OSL calibration
In the medical field, the only OSL system currently available is the nanoDot® (Landauer, Inc., Glenwood, IL) (Figure 2). It composed of a carbon-doped Aluminum Oxide (Al2O3:C) crystal, 4 mm in diameter and 0.2 mm thick, encapsulated in a light-opaque plastic housing measuring 10 × 10 × 2 mm. This detector enables point dose measurements by converting the optical response (M_corr) into absorbed dose in water (D_w) using the equation:
D_w = N_D,w × M_corr × k_L × k_F × k_Q × k_θ × k_s,i (1)
Fig. 2. OSL.
T he calibration coefficient N_{D,w} is defined as the ratio of the known dose D_0 to the corresponding corrected detector reading M_{0,corr}. Only the angular correction factor k_θ is applied, as stable calibration conditions allow all other correction factors to be set to 1.
Calibration was conducted with a 6 MV photon radiation beam. (10 × 10 cm² field, 100 cm SSD) with a PMMA phantom (1.5 cm build-up, 10 cm backscatter). OSLs from the same batch were placed on a 1 cm Superflab slab for uniformity. Five detectors were irradiated on and off-axis at 100, 200 and 300 MU per session, with 10 OSLDs tested per dose level. Dose measurements were made using an IAEA-calibrated PTW-30113 ionization chamber (calibration factor: 53.52 ± 0.54 mGy/nC). The final calibration factor using the microStar® reader was 856.472 counts/cGy. Coefficients of variation (0.024, 0.032, 0.024) were below the 0.05 limit, confirming the nanoDot® system's high reproducibility and reliability.
Treatment planning and setup
T he phantom was positioned supine on the treatment couch, with alignment performed using in-room laser systemsA CT scan for treatment setup with a 2.5 mm slice thickness was performed and imported into the Eclipse treatment planning system (version 18.00.10, Varian Medical Systems, Palo Alto, CA). Two distinct radiotherapy techniques were planned: IMRT was delivered using seven different beam angles with a sliding window technique and VMAT was performed with dual arcs, one in the Clockwise (CW) and one in the Counterclockwise (CCW) direction. Both plans used 6 MV photon beams and treatment was simulated using a Varian TrueBeam linear accelerator. Target volumes and organs at risk were contoured in accordance with the ICRU Report 38 [10]. T he prescribed dose was 46Gy in 23 fractions of 2 Gy, with dose calculations performed using the Acuros XB_18.0.1 Algorithm (with heterogeneity correction enabled).
Dosimeter placement
Eight OSL dosimeters were affixed to the surface of a female anthropomorphic phantom using transparent adhesive tape at eight clinically relevant beam entry sites (Figure 3). The dosimeters were placed at the following anatomical landmarks: anterior (pubic symphysis), posterior (sacral midline), right lateral (right iliac crest), and left lateral (left iliac crest). Additional placements included the Right Anterior Oblique (RAO) and Left Anterior Oblique (LAO), positioned at the right and left lower abdominal quadrants, respectively each midway between the pubic symphysis and the corresponding iliac crest. The Right Posterior Oblique (RPO) and Left Posterior Oblique (LPO) dosimeters were placed over the gluteal regions, each located midway between the sacrum and the corresponding iliac crest. These locations were selected to capture regional variability of skin dose in high-gradient areas and beam entry points, while two sites were instrumented in duplicate to evaluate repeatability and repositioning uncertainty. The final configuration of eight detectors represented a balance between spatial coverage, statistical precision (through reduction of standard error), and feasibility (in terms of placement and reading time), thereby ensuring a robust comparison of IMRT–VMAT surface dose distributions.
Fig. 3. Placement of eight OSL dosimeters on the phantom.
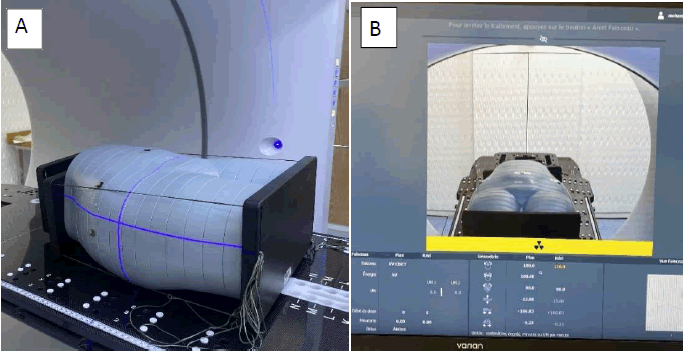
Positioning was verified using Cone Beam Computed Tomography (CBCT) [11] to ensure proper and reproducible phantom posi tioning as well as accurate dosimeter alignment with the incident beams for both IMRT and VMAT deliveries (Figure 4A).
T he phantom was irradiated using both IMRT and VMAT plans in separate sessions (Figure 4B). After each exposure, nanoDots were removed and read within 24 hours to minimize signal loss due to fading.
Fig. 4. (A) Positioning procedure and (B) CBCT imaging acquisition.
Data analysis and radiation protection
Percentage deviations between measured and TPS-calculated doses were computed for each location and technique. The results were evaluated in the context of international radiation protection standards, particularly those outlined by the International Commission on Radiological Protection [12]. All measurements were assessed for compliance with the ALARA (As Low as Reasonably Achievable) principle and findings were used to verify the accuracy of the TPS in surface dose prediction.
Results
Measured skin entrance doses
T he measured entrance doses using OSLs at the eight anatomical locations on the female anthropomorphic phantom are summarized in Table 1. Results are shown for both IMRT and VMAT techniques. For each technique, the entrance dose was measured in centiGray (cGy) and compared to the corresponding TPS-calculated surface dose values in Gray (Gy).
| Location | VMAT measured dose (mean) | VMAT TPS dose | ΔVMAT (%) | IMRT measured dose (mean) | IMRT TPS dose | ΔIMRT (%) |
| Anterior | 0.2936 | 0.286 | 2.70% | 0.301 | 0.29 | 3.80% |
| Posterior | 0.548 | 0.535 | 2.40% | 0.587 | 0.559 | 5.00% |
| Right lateral | 0.548 | 0.528 | 3.80% | 0.7199 | 0.685 | 5.10% |
| Left lateral | 0.505 | 0.494 | 2.20% | 0.596 | 0.575 | 3.60% |
| RAO | 0.513 | 0.489 | 4.90% | 0.551 | 0.528 | 4.40% |
| LAO | 0.645 | 0.618 | 4.30% | 0.521 | 0.498 | 4.60% |
| RPO | 0.381 | 0.372 | 2.40% | 0.527 | 0.513 | 2.70% |
| LPO | 0.5201 | 0.496 | 4.80% | 0.916 | 0.881 | 4.00% |
| Mean | 0.494 | 0.477 | 3.56% | 0.589 | 0.566 | 4.06% |
| OTD | 11.362 | 10.971 | 3.56% | 13.547 | 13.018 | 4.06% |
| Note: Δ=(Measured-TPS)/TPS × 100; RAO: Right Anterior Oblic; LAO: Left Anterior Oblic; RPO: Right Posterior Oblic; LPO: Left Posterior Oblic; OTD: Overall Treatment Dose=Dose per fraction × 23 fractions | ||||||
Tab. 1. Comparison of measured and TPS-calculated skin entrance doses (in Gy).
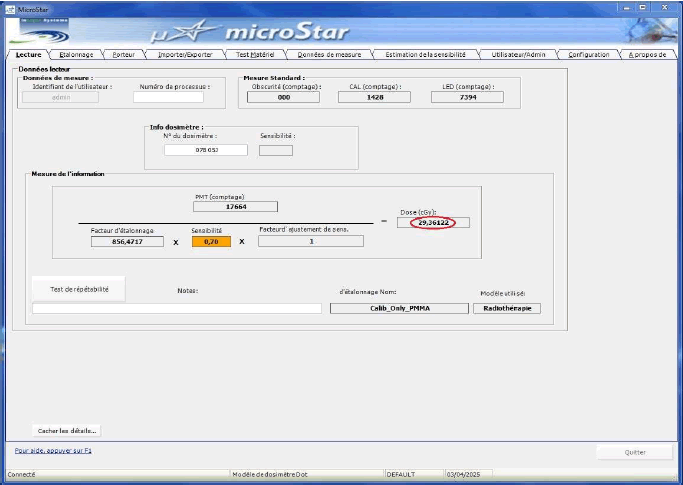
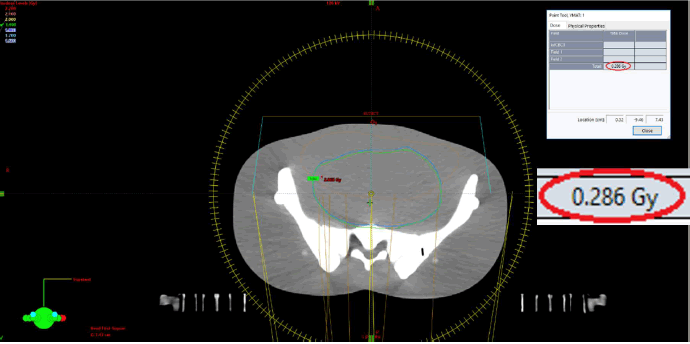
Dose assessments were carried out at the front surface of the phantom in VMAT techniques, specifically at the level of the pubic symphysis, a region exhibiting a steep dose gradient. The dose recorded using the OSLs was 0.2936 Gy (Figure 5), while the corresponding dose calculated by the TPS was 0.286 Gy (Figure 6), yielding a relative difference of approximately +2.7%.
Fig. 5. Reading of OSLs using the MicroStar reader.
Fig. 6. Dose calculation by TPS in VMAT.
Dose agreement
For both techniques, measured doses were in good agreement with TPS calculated values, with percentage deviations ranging between +2.2% and +5.1% (Table 1). Slightly higher entrance doses were observed with IMRT at most locations except for the anterior.
Technique comparison: VMAT vs. IMRT
IMRT demonstrated slightly higher skin entrance doses at all measured points compared to VMAT except for the anterior position. The average entrance dose across all points was 0.494 Gy for the measured dose and 0.477 Gy for the calculated dose with VMAT, while for IMRT, the measured dose was 0.589 Gy and the calculated dose was 0.566 Gy. The relative increase in entrance dose with IMRT was approximately 0.5% on average (Table 1).
Discussion
To analyze and compare the OSL-measured doses and TPS calculated doses for IMRT. The highest OSL measured dose was observed at the left posterior oblique position 0,916 Gy, as shown in Figure 7, while the lowest was at the anterior position 0,301 Gy, with an overall average dose of approximately 0,589Gy. In contrast, TPS-calculated doses ranged from 0,290 Gy (anterior) to 0,881Gy (LPO), with a slightly lower average of 0,566 Gy. The comparison revealed that OSL readings were consistently higher than TPS values across all measurement points, with an average absolute difference of 0,023 Gy.
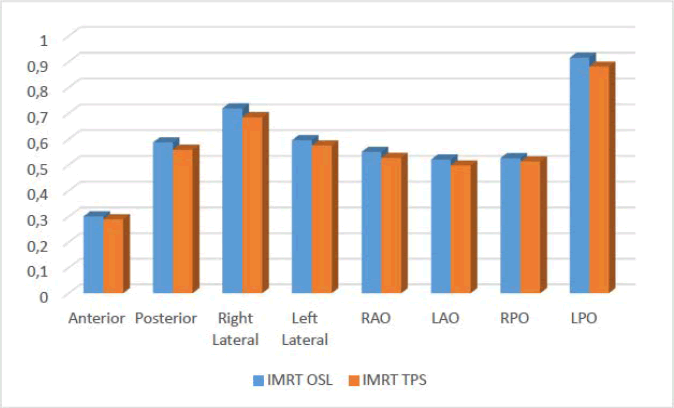
Fig. 7. Comparison of measured and calculated Doses for IMRT.
T he largest discrepancy 0,035 Gy occurred at the LPO, a region often exposed to complex beam angles and tissue heterogeneity, which may contribute to inaccuracies in dose modeling by the TPS. The smallest difference 0,011Gy was at the anterior position, suggesting better TPS agreement in posterior regions with less anatomical complexity.
T he comparison between the measured and TPS doses for the VMAT technique (Figure 8) reveals a generally consistent alignment, with measured doses being slightly higher across all angles. The differences, ranging from 0,0076 Gy (at Anterior) to 0,027 Gy (at LAO), indicate a minor discrepancy between the actual delivered dose and the planned dose, suggesting that the TPS may slightly underestimate the radiation dose in certain directions. These small variations could be attributed to several factors specific to VMAT delivery, including uncertainties in patient positioning, intricacies in the dynamic motion of the multileaf collimator or inaccuracies in the dose calculation algorithms used by the TPS.

Fig. 8. Comparison of measured and calculated doses for VMAT.
An important factor to consider in the evaluation of radiotherapy techniques is the influence of tissue heterogeneity on dose distribution. The phantom comprises a wide range of tissue types, each with distinct densities and compositions. These variations can significantly affect how radiation is absorbed and dispersed, particularly in advanced techniques like VMAT. Therefore, tissue heterogeneity may be a key contributor to the dosimetric differences observed between two techniques in clinical practice.
When comparing IMRT and VMAT in the context of cervical cancer treatment, both modalities are effective in achieving accurate dose conformity and target coverage. However, one recurrent observation is that IMRT tends to deliver a slightly higher entrance dose to the skin than VMAT. This difference warrants closer examination, as it has implications for skin toxicity at the pelvic region and overall treatment safety. The explanation lies in the fundamental delivery mechanics of the two techniques. VMAT operates by continuously rotating the linear accelerator around the patient, delivering radiation in an arc trajectory from a wide range of angles. This dynamic and highly conformal approach allows for shorter overall treatment times and more efficient dose distribution, which can lead to lower entrance skin doses. The rotational delivery spreads the dose over a larger skin surface area, reducing the intensity at any single point, thereby minimizing localized skin exposure.
In contrast, IMRT uses multiple static fields from fixed angles, each modulating the intensity of the beam. While effective, this technique typically requires longer treatment times and may concentrate dose deposition along fewer skin entry points, potentially increasing entrance skin dose in those regions. Therefore, VMAT offers advantages in terms of both reduced skin exposure time and improved spatial distribution of the radiation dose, making it a more efficient and skin-sparing technique compared to IMRT. T herefore, from a dosimetric standpoint, VMAT may offer a more favorable profile with respect to skin sparing, particularly due to its ability to distribute dose more evenly across the skin surface and reduce treatment time [13]. This highlights the importance of considering not only target conformity but also beam delivery dynamics and efficiency when selecting the optimal radiotherapy technique. While IMRT can be effective, it typically involves longer treatment times and delivers radiation from fewer fixed angles, which can concentrate dose at specific skin entry points [14]. In contrast, VMAT’s superior dose conformity, enhanced sparing of organs at risk and reduced exposure time contribute to its clinical advantages. The minor variations in entrance skin dose are generally not significant and are outweighed by VMAT’s overall efficiency, precision and improved patient experience, making it a highly attractive option in modern radiotherapy practice.
In our study, the measured skin doses were 11.362 Gy with VMAT and 13.547 Gy with IMRT, representing only 24.7% and 29.4% of the 46 Gy prescribed dose, respectively. These values fall well below the established thresholds for acute skin toxicity, which typically begins above 20 Gy and for chronic effects, which occur beyond 40 Gy [15]. This indicates a low risk of both acute and chronic cutaneous reactions. Notably, VMAT delivered a lower skin dose than IMRT, reinforcing its advantage in skin sparing. T hese findings support the use of VMAT as the preferred technique to minimize skin toxicity in cervical cancer radiotherapy.
However, in cases where patients have skin sensitivity or pre existing skin problems, the increased skin dose with IMRT could be a concern. In such situations, VMAT might be preferred due to its ability to limit skin exposure, thus minimizing the risk of skin irritation or damage. Ultimately, the choice between IMRT and VMAT should take into consideration not only the dosimetric advantages of each technique but also the patient's individual needs and sensitivities, especially when skin-related issues are a concern.
Despite these minor variations, the overall agreement between the measured and TPS doses suggests that the VMAT treatment planning system is performing within an acceptable margin of error. However, regular verification and quality assurance processes are essential to ensure that the planned doses are accurately delivered, minimizing potential risks to healthy tissues while maximizing therapeutic outcomes. Further investigation could examine the clinical significance of these discrepancies, particularly in areas receiving high doses. Another reason to consider is the arc length in treatment planning is its significant impact on skin dose distribution; longer arcs reduce low-grade skin reactions, while shorter and full arcs increase high-grade reactions due to overlapping entrance and exit doses, making arc length as crucial as PTV to skin distance in minimizing skin toxicity [16]. This underlines the importance of careful VMAT planning to mitigate unnecessary entrance dose, especially for treatment regions like the pelvis.
T his study shows strong alignment with ICRU Reports 50 and 83 [17,18], which recommend that the dose to the clinical target volume remain within ± 5% of the prescribed dose. The OSL measured and TPS-calculated doses demonstrated high dosimetric accuracy, with an average deviation of 4.15% in IMRT and 3.44% in VMAT. The maximum deviation observed was 5,1% in IMRT slightly exceeding ICRU limits but still considered clinically acceptable [18]. Consistent with the study of Vinh [19], who demonstrated that optimized IMRT plans significantly reduce high dose exposure to the skin in pelvic cancer patients, our findings validate the accuracy of TPS and highlight the potential of OSL dosimetry as a reliable quality assurance tool. T hese results validate TPS accuracy and support the use of OSL dosimetry as a reliable QA (Quality Assurance) tool. The OSLs, with high spatial resolution and minimal energy dependence [20] meet ICRU standards for surface dose measurement in VMAT and IMRT. However, limitations such as phantom-based modeling and potential angular dependence [21] underscore the need for further refinement in clinical settings.
While the research contributes important knowledge, some limitations warrant attention, such as the phantom based approach, which, while anatomically accurate, does not fully account for physiological factors like skin elasticity, moisture and movement in live patients [22]. Though precise, the OSLs may still have limitations in angular dependency at very steep beam incidences. T he adhesive used to secure OSL (e.g. Scotch tape) could also slightly affect scatter conditions, although this impact is minimal. Furthermore, the study is limited to a single anatomical site pelvis (cervical cancer) and two planning techniques, which may not generalize across all treatment sites and modalities. In addition, the evaluation of integral dose was not included, which could provide further insight into the total energy deposited in normal tissues and its long-term implications [23]. Although OSLDs provide only point dose measurements and Eclipse TPS may slightly underestimate skin dose due to electronic contamination and build-up, these findings still offer valuable comparative insights. T he limited number of measurement points does not capture the full heterogeneity of pelvic skin dose; nonetheless, the data effectively highlight key trends and inform future strategies for more comprehensive dose assessment.
Conclusion
T his study confirms that VMAT delivers lower skin doses than IMRT in cervical cancer radiotherapy, reducing the risk of acute and chronic skin toxicity. Both techniques stayed within ICRU recommended dose limits, with VMAT showing better skin sparing and treatment efficiency. The minor deviations between measured and planned doses validate TPS accuracy and support OSL nanoDots as a reliable QA tool. VMAT's advantages in dose conformity, reduced treatment time and lower skin exposure make it the preferred technique, particularly for patients with skin sensitivity.
Acknowledgments
T he authors extend their heartfelt appreciation to Mr. Mohamed Abour, Medical Physicist at the Radiotherapy Department, International Oncology Center of Benguérir, Morocco, for his generous support and technical assistance. Gratitude is also due to the entire staff of the center for their cooperation and commitment during the course of this work. Special thanks to Professor Mohamed Hilal, my supervisor of this work, for his continuous guidance and encouragement.
Funding
T he authors declare no financial support was received.
Conflicts of Interest
No conflicts of interest are declared regarding this work.
Authors Contribution
T he study was conceived and designed, data were collected and analyzed, and the findings interpreted and drafted into a manuscript. Assistance with data collection and analysis was provided, and the study was supervised with critical review of the manuscript. All authors read and approved the final version: Ahlam Azalmad, Abour Mohamed, Mohamed Hilal.
References
- Hull R, Mbele M, Makhafola T, Hicks C, Wang SM, et al. Cervical cancer in low and middleâÂÂÂÂincome countries. Oncol Lett. 2020; 20:2058-2074.
[Crossref] [Google Scholar] [PubMed]
- Mayadev JS, Ke G, Mahantshetty U, Pereira MD, Tarnawski R, et al. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int J Gynecol Cancer. 2022; 32:436-445.
[Crossref] [Google Scholar] [PubMed]
- Azalmad A, Nhila O, Hilal M. New study on optimizing cervical cancer treatment: Dosimetric Comparison of 3D-CRT, IMRT and VMAT techniques at Beni Mellal oncology center. J Obstet Gynecol Cancer Res. 2025.
- Azalmad A, Hilal M, Elmaadaoui Y. Advancing precision in cervical cancer radiotherapy: Assessing Setup Errors and Adjusting PTV Margins. J Obstet Gynecol Cancer Res. 2025.
- Yeung AW. The'As Low as Reasonably Achievable'(ALARA) principle: A brief historical overview and a bibliometric analysis of the most cited publications. Radioprotection. 2019.
- MrÃÂÂÂela I, BokuliÃÂÂÂ T, Izewska J, Budanec M, Fröbe A, et al. Optically stimulated luminescence in vivo dosimetry for radiotherapy: physical characterization and clinical measurements in 60Co beams. Phys Med Biol. 2011; 56:6065.
[Crossref] [Google Scholar] [PubMed]
- Lu MY, Ting CY, Jao JC. Effective dose and radiation risk under 640-slice abdominal computed tomography examination without contrast medium injection. J X-ray Sci Technol. 2022; 30:657-666.
[Crossref] [Google Scholar] [PubMed]
- IAEA. Dosimetry in Diagnostic Radiology: An International Code of Practice. Technical Reports Series No. 457. IAEA, Vienna. 2007.
- CIRS. ATOM® Dosimetry Phantoms Models 701–706. Product brochure. 2018.
- Bellotti JE, Kagan AR, Wollin M, Olch A. Application of the ICRU Report 38 reference volume concept to the radiotherapeutic management of recurrent endometrial and cervical carcinoma. Radiother Oncol. 1993; 26:254-259.
[Crossref] [Google Scholar] [PubMed]
- de Crevoisier R, Garcia R, Louvel G, Marguet M, Lafond C, et al. Cone beam computed tomography-guided radiotherapy: Implementation and clinical applications. Cancer/Radiotherapy. 2009; 13:482-489.
- Protection R. ICRP publication 103. Ann Icrp. 2007; 37:2.
- Wu Y, Zhu B, Han J, Xu H, Gong Z, et al. A comparative dosimetric study of cervical cancer patients with para-aortic lymph node metastasis treated with volumetric modulated arc therapy vs. 9-field intensity-modulated radiation therapy. Ann Transl Med. 2019; 7:675.
[Crossref] [Google Scholar] [PubMed]
- Penoncello GP, Ding GX. Skin dose differences between intensity-modulated radiation therapy and volumetric-modulated arc therapy and between boost and integrated treatment regimens for treating head and neck and other cancer sites in patients. Med Dosim. 2016; 41:80-86.
[Crossref] [Google Scholar] [PubMed]
- Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther. 2016; 6:185-206.
[Crossref] [Google Scholar] [PubMed]
- Rijken J, Kairn T, Crowe S, Trapp J. Effect of arc length on skin dose from hypofractionated volumetric modulated arc radiotherapy treatments of the lung and spine. Med Dosim. 2019; 44:309-314.
[Crossref] [Google Scholar] [PubMed]
- ICRU. Prescribing, Recording and Reporting Photon Beam Therapy. ICRU Report No. 50. Bethesda, MD: ICRU; 1993.
- ICRU. Prescribing, Recording and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT). ICRU Report No. 83. J ICRU. 2010; 10:1-106.
- Vinh QB, Quoc SD, Van TH, Vu T, Thi TP. Reduction of the skinâÂÂÂÂeffect dose of IMRT plan for patients with cancer in pelvic region. Mol Clin Oncol. 2023; 18:43.
[Crossref] [Google Scholar] [PubMed]
- Jursinic PA. Characterization of optically stimulated luminescent dosimeters, OSLDs, for clinical dosimetric measurements. Med Phys. 2007; 34:4594-4604.
[Crossref] [Google Scholar] [PubMed]
- Kerns JR, Kry SF, Sahoo N, Followill DS, Ibbott GS. Angular dependence of the nanoDot OSL dosimeter. Med Phys. 2011; 38:3955-3962.
[Crossref] [Google Scholar] [PubMed]
- Kaatee RS, Olofsen MJ, Verstraate MB, Quint S, Heijmen BJ. Detection of organ movement in cervix cancer patients using a fluoroscopic electronic portal imaging device and radiopaque markers. Int J Radiat Oncol Biol Phys. 2002; 54:576-583.
[Crossref] [Google Scholar] [PubMed]
- Yeh CY. Integral dose comparison of VMAT and IMRT for rectal cancer treatment: A dosimetric analysis. Radiat Phys Chem. 2024; 221:111736.