Case Report - Onkologia i Radioterapia ( 2025) Volume 19, Issue 12
Durable Response to RibociclibâFulvestrant in Breast Cancer With Pancreatic Metastasis and Neuroendocrine Features: A Case Report
Nejjari Sara*, Keita Diango, Alem Mehdi, Oufrid Abir, El Hilali Hafssa, Chbihi Chaymae, El Hakym Samia, Aabboub Basma, Amaadour Lamiae, Oualla Karima, Benbrahim Zineb, Arifi Samia, Mayeur Didier and Mellas NawfelMorocco
Morocco
Morocco
Morocco
Morocco
Morocco
Morocco
2Morocco
3Morocco
4Morocco
Morocco
5Morocco
6Morocco
7Morocco
Nejjari Sara, Department of Medical Oncology, Oncology Hospital, Hassan II University Hospital, Faculty of Medicine, Pharmacy and Dentistry, Fez, Morocco, Email: sara.nejjari@usmba.ac.ma
Received: 01-Dec-2025, Manuscript No. OAR-25-176342; , Pre QC No. OAR-25-176342 (PQ); Editor assigned: 03-Dec-2025, Pre QC No. OAR-25-176342 (PQ); Reviewed: 19-Dec-2025, QC No. OAR-25-176342; Revised: 24-Dec-2025, Manuscript No. OAR-25-176342 (R); Published: 31-Dec-2025
Abstract
Invasive breast carcinoma with neuroendocrine differentiation (BC-NED) is a rare and distinct subtype. The combination of a rare metastatic site like the pancreas and a rare tumor subtype, as seen in our patient, presents a unique clinical scenario. We report the case of a 53-year-old female who was initially treated in 2011 for an HR+/HER2+ invasive ductal carcinoma of the right breast. After receiving adjuvant therapy, the patient developed a lesion in the pancreatic head in February 2020. Histopathological analysis confirmed the presence of a carcinoma consistent with a breast origin, but with the emergence of a neuroendocrine component. Systemic treatment with ribociclib plus fulvestrant was initiated. The patient achieved a complete clinical and radiological metabolic response with this regimen, which has been maintained to date with good treatment tolerance. The prognosis for patients with pancreatic metastases is generally poor, which makes our patient's exceptional response particularly intriguing. The de-novo emergence of the neuroendocrine component during metastasis is a fascinating aspect of this case, suggesting a potential clonal evolution. While most trials of CDK4/6 inhibitors excluded patients with neuroendocrine tumors, this case demonstrates the potential for their use in this setting. This case report illustrates that breast cancer with a neuroendocrine component, even in the context of visceral metastasis, can have a durable and favorable response to targeted endocrine therapy in combination with a CDK4/6 inhibitor. This finding highlights the importance of a thorough pathological and molecular evaluation to guide treatment decisions.
Keywords
Cdk4/6 Inhibitor; Hormone Receptor–Positive Breast Cancer; Neuroendocrine Breast Carcinoma; Pancreatic Metastasis; Ribociclib and Fulvestrant
INTRODUCTION
Invasive breast carcinoma with neuroendocrine differentiation (BC-NED) is a rare and distinct subtype, representing less than 5% of all breast cancers [1, 2]. The World Health Organization (WHO) classifies these tumors into well-differentiated neuroendocrine tumors (NET), poorly differentiated neuroendocrine carcinomas (NEC), and invasive breast carcinoma with neuroendocrine differentiation [3]. This subtype is often associated with a higher grade, larger tumor size, and a less favorable prognosis compared to conventional invasive ductal carcinoma (IDC) [1,4]. While endocrine receptors are frequently positive, the optimal therapeutic approach remains uncertain, particularly in the metastatic setting [5].
Metastasis to the pancreas is uncommon, occurring in less than 5% of breast cancer patients [6,7]. Pancreatic involvement usually indicates aggressive disease and poses significant diagnostic and therapeutic challenges. The combination of a rare metastatic site and rare tumor histology, as observed in our patient, creates an exceptional clinical scenario. We report the case of a 53-year-old woman with HR-positive/HER2-positive IDC who developed pancreatic metastasis containing a neuroendocrine component. She achieved a long-term and ongoing response to first-line ribociclib plus fulvestrant, challenging the expected behavior of such tumors.
Case Presentation
A 53-year-old woman with a history of breast cancer was initially diagnosed in 2011 with right-sided invasive ductal carcinoma located at the upper-quadrant junction. Pathology showed pT2 (25 mm), pN1 (1/15), M0, SBR grade 2, ER-positive, and HER2 3+. She underwent breast-conserving surgery followed by adjuvant chemotherapy (six cycles of docetaxel-trastuzumab), trastuzumab maintenance until July 2012, adjuvant radiotherapy, and endocrine therapy with tamoxifen. Tamoxifen was discontinued after two years due to recurrent phlebitis. In September 2014, she underwent bilateral adnexectomy and began letrozole, which was soon discontinued because of severe arthralgia. In February 2020, she presented with pruritic jaundice related to a pancreatic head mass complicated by acute pancreatitis. Histopathological analysis of the pancreatic mass confirmed metastatic carcinoma with neuroendocrine differentiation, with an immunophenotype consistent with breast origin (ER-positive, PR-negative, HER2-negative) [Figure 3]. A CT scan on March 5, 2020, showed a slight expansion of the pancreatic tumor [Figure 1].
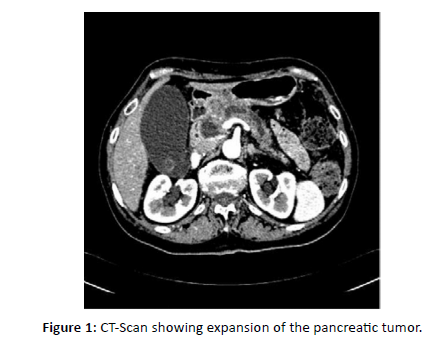
Figure 1: CT-Scan showing expansion of the pancreatic tumor.
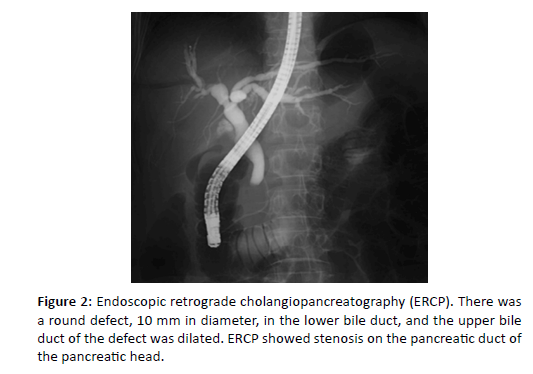
Figure 2: Endoscopic retrograde cholangiopancreatography (ERCP). There was a round defect, 10 mm in diameter, in the lower bile duct, and the upper bile duct of the defect was dilated. ERCP showed stenosis on the pancreatic duct of the pancreatic head.
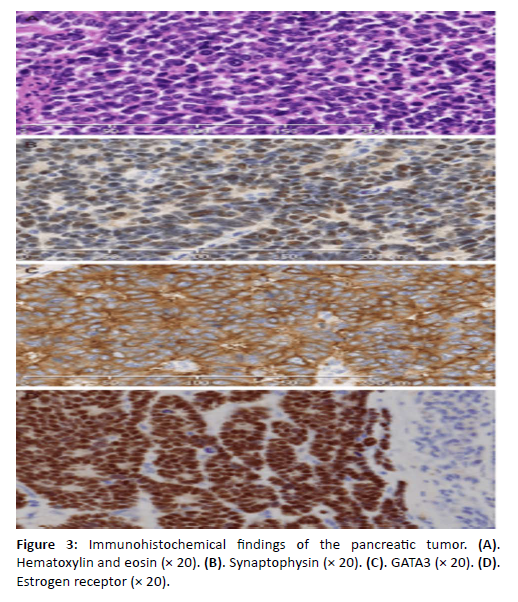
Figure 3: Immunohistochemical findings of the pancreatic tumor. (A). Hematoxylin and eosin (Ã 20). (B). Synaptophysin (Ã 20). (C). GATA3 (Ã 20). (D). Estrogen receptor (Ã 20).
Endoscopic retrograde cholangiopancreatography (ERCP) [Figure 2], revealed a round defect, 10 mm in diameter, in the intrapancreatic bile duct, and the upper bile duct of the defect was dilated. ERCP showed stenosis on the pancreatic duct of the pancreatic head [Figure 2].
Systemic therapy with ribociclib and fulvestrant was initiated on May 11, 2020. The patient achieved a complete clinical and metabolic radiologic response that has been maintained to the present, with excellent tolerance.
DISCUSSION
Breast cancer typically metastasizes to the bone, liver, and lung. Pancreatic involvement is exceedingly rare, with an incidence of solitary pancreatic metastasis reported at <3%. A review of the literature identified only 11 documented cases of isolated pancreatic metastases from breast cancer [1]-[8], most frequently affecting the pancreatic head rather than the tail. Among these cases, invasive lobular carcinoma was the predominant histological subtype (8 cases), with isolated reports of signet-ring cell, comedo, and scirrhous carcinomas. The disease-free interval reported in previous cases ranges from 16 to 84 months (mean 43.3 months), consistent with our patient’s interval of 24 months, who remained asymptomatic during this period [1]-[9]. Although ultrasonography, CT scan, and MRI are routinely used to evaluate pancreatic lesions, differentiating metastatic involvement from a primary pancreatic neoplasm remains challenging [10]. Pancreatic biopsy is the most reliable diagnostic tool, yet it may be inconclusive or technically unfeasible. Since metastatic breast lesions can mimic primary gastrointestinal cancers, serum CA 15-3 may aid in distinguishing the two, although elevations are not consistently observed. Nagao et al. [11], documented that pancreatic metastases from breast cancer are extremely uncommon, with only 20 resected cases reported in the literature-a figure that underscores the rarity of this metastatic pattern and supports the exceptional nature of our case. In their review, they observed that most patients present with metachronously pancreatic metastasis, with intervals ranging from 2 months to 26 years after initial breast cancer diagnosis, demonstrating the wide temporal variability of metastatic spread in breast malignancies. They also emphasized that preoperative diagnosis is rarely achieved, as only two cases in the literature were correctly identified as pancreatic metastasis prior to surgery, highlighting the significant diagnostic challenges posed by these lesions even when advanced imaging is available. Moreover, Nagao et al. [11], reported that long-term survival is possible after resection, with one patient remaining disease-free for more than 15 years, suggesting that carefully selected patients with isolated pancreatic involvement may benefit from aggressive local therapy when systemic disease is controlled. Pancreatic metastasis from breast cancer has been described only exceptionally, and the case reported by Tohnosu et al. [1], represents the seventh documented case in the literature and the first involving the pancreatic tail, highlighting the unusual metastatic pattern observed in their patient. Unlike most previously published cases-which predominantly involved the pancreatic head and frequently presented with obstructive jaundice-Tohnosu et al.’s patient was asymptomatic, and the lesion was detected only after a progressive rise in CA15-3 and TPA, illustrating how metastatic spread may remain clinically silent despite biochemical progression.
Histologically, the metastasis mimicked the primary breast tumor, and immunohistochemical positivity for CA15-3 and GCDFP-15 confirmed its mammary origin, reinforcing the diagnostic value of breast-specific markers when imaging findings are non-specific. Furthermore, the authors emphasized that only six prior solitary pancreatic metastases had been fully described, underscoring the extreme rarity of such presentations and the difficulty of establishing a preoperative diagnosis. Their case also demonstrated that long-term control is feasible when systemic disease is otherwise limited, as their patient remained alive under paclitaxel plus aromatase inhibitor therapy, despite subsequent osseous progression. Collectively, these findings parallel our case by highlighting the diagnostic challenges, the potential role of serum markers, and the importance of integrating imaging and immunohistochemistry for correct identification of pancreatic metastasis from breast carcinoma. Only 10 breast cancer patients who underwent resection of pancreatic metastases [Table 1], have been reported [1],[2],[5],[6],[7],[9],[12]-[20]. However, only two patients were simultaneously diagnosed with pancreatic and breast cancer. They underwent simultaneous pancreaticoduodenectomy (PD) and mastectomy after being diagnosed with papilla of Vater and pancreatic head cancers, respectively [12, 14]. In the remaining 18 cases, pancreatic tumors were diagnosed metachronously with intervals ranging between 2 months and 26 years after breast cancer resection. Moreover, no reported case underwent neoadjuvant chemotherapy before pancreatectomy. Prognosis for patients with pancreatic metastases appears more favorable than for primary pancreatic carcinoma [9]. However, therapeutic strategies remain debated. Surgical resection has been proposed in selected cases, including metastases from breast cancer [6], [9], but pancreatic surgery carries substantial morbidity and a non-negligible mortality rate [7]. Consequently, resection should be considered only when the metastatic lesion is isolated to the pancreas and technically respectable [6].
| Case no | Ref | Age (Y.O) | Timing | Interval (months) | Preop diag | Preoperative or intraoperative diagnosis of pancreatic tumor | Outcome | Duration (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | [7] | 52 | Metachronous | 38 | Pancreatic head cancer | Pancreaticoduodenectomy | Alive | 72 |
| 2 | [2] | 33 | Metachronous | 30 | Pancreatic head cancer | Pancreaticoduodenectomy | Alive | 27 |
| 3 | [5] | 46 | Metachronous | 80 | Cancer of the papilla of Vater | Pancreaticoduodenectomy | Alive | 12 |
| 4 | [6] | 70 | Metachronous | 36 | Pancreatic head tumor | Pylorus-preserving pancreaticoduodenectomy | Alive | 37 |
| 5 | [6] | 46 | Metachronous | 60 | Pancreatic head tumor | Pylorus-preserving pancreaticoduodenectomy | Alive | 21 |
| 6 | [6] | 57 | Metachronous | 84 | Pancreatic head tumor | Pylorus-preserving pancreaticoduodenectomy | Dead | 26 |
| 7 | [12] | 75 | Metachronous | 312 | Pancreatic head tumor | Enucleation | Alive | 80 |
| 8 | [1] | 57 | Metachronous | 54 | Metastatic pancreatic tumor from breast cancer | Distal pancreatectomy | Alive | 5 |
| 9 | [9] | 60 | Metachronous | 2 | Bile duct cancer | Pancreaticoduodenectomy | Alive | 2 |
| 10 | [9] | 55 | Metachronous | 114 | Pancreatic tail tumor (adenocarcinoma was confirmed by frozen section) | Distal pancreatectomy | Alive | 2 |
| 11 | [13] | 41 | Metachronous | 60 | Metastatic pancreatic tumor from breast cancer | Distal pancreatectomy | Alive | |
| 12 | [14] | 53 | Metachronous | 20 | Pancreatic head cancer | Pancreaticoduodenectomy | Dead | 36 |
| 13 | [15] | 75 | Metachronous | 216 | Pancreaticoduodenectomy | Alive | 48 | |
| 14 | [16] | 68 | Simultaneous | 0 | Cancer of the papilla of Vater | Pancreaticoduodenectomy | Alive | 12 |
| 15 | [17] | 34 | Metachronous | 19 | Pancreaticoduodenectomy | Alive | 12 | |
| 16 | [18] | 48 | Simultaneous | 0 | Pancreatic head cancer | Pancreaticoduodenectomy | Dead | 12 |
| 17 | [19] | Pancreaticoduodenectomy | Alive | |||||
| 18 | [20] | 35 | Metachronous | 45 | Dead | 7 | ||
| Present case | 53 | Metachronous | 96 | Metastatic pancreatic tumor from breast cancer | Alive | 60 |
Table 1: Reported Cases Undergoing Pancreatic Metastasis Resection From Breast Cancer
CONCLUSION
This case report illustrates an exceptional response to ribociclib and fulvestrant in a patient with a rare presentation of metastatic breast cancer. This report adds to the growing body of literature supporting the use of CDK4/6 inhibitors in complex metastatic breast cancer and suggests that the presence of a neuroendocrine component, particularly with a low Ki-67 index, should not be a definitive barrier to endocrine-based treatment strategies.
References
- Tohnosu N, Narushima K, Sunouchi K. A case of breast cancer metastatic to the tail of the pancreas. Breast Cancer. 2006; 13: 225 229. [Crossref], [Google Scholar], [PubMed]
- Mehta SA, Jagannath P, Krishnamurthy SC. Isolated pancreatic metastasis from locally controlled breast cancer: a case report. mars 1991; 28: 48-50. [Google Scholar], [PubMed]
- Mountney J, Maury AC, Jackson AM. Pancreatic metastases from breast cancer: an unusual cause of biliary obstruction. Eur J Surg Oncol. 1997; 23: 574-576. [Crossref], [Google Scholar], [PubMed]
- Engel JJ, Trujillo Y, Spellberg M. Metastatic carcinoma of the breast: a cause of obstructive jaundice. Gastroenterology. 1980; 78: 132-135. [Google Scholar], [PubMed]
- Nomizu T, Katagata N, Matsuoka T. A Case of Breast Cancer Metastatic to the Head of the Pancrea. Breast Cancer. 1999; 6: 131-134. [Crossref], [Google Scholar], [PubMed]
- Crippa S, Bonardi C, Bovo G. Pancreaticoduodenectomy for pancreatic metastases from breast carcinoma. JOP. 2004; 5: 377-383. [Google Scholar], [PubMed]
- Azzarelli A, Clemente C, Quagliuolo V. A case of pancreatoduodenectomy as resolutive treatment for a solitary metastasis of breast cancer. Tumori. 1982; 68: 331-335. [Crossref], [Google Scholar], [PubMed]
- Hiotis SP, Klimstra DS, Conlon KC. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002; 9: 675-679. [Crossref], [Google Scholar], [PubMed]
- Hernáez O, Fernández. Pancreatic metastases from ductal and lobular carcinomas of the breast. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2007; 9: 603-605. [Crossref], [Google Scholar], [PubMed]
- Kidney DD, Cohen AJ, Butler J. Abdominal metastases of infiltrating lobular breast carcinoma: CT and fluoroscopic imaging findings. Abdom Imaging. 1997; 22: 156-159. [Crossref], [Google Scholar], [PubMed]
- Nagao A, Noie T, Horiuch H. Long-term survival after pancreatic metastasis resection from breast cancer: a systematic literature review. Surg case rep 2021; 7: 39. [Crossref], [Google Scholar], [PubMed]
- Minni F, Casadei R, Perenze B. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology 2004; 4: 509-520. [Crossref], [Google Scholar], [PubMed]
- Sweeney AD, Wu MF, Hilsenbeck SG. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2009; 156: 189-198. [Crossref], [Google Scholar], [PubMed]
- Bonapasta SA, Gregori M, Lanza R. Metastasis to the Pancreas from Breast Cancer: Difficulties in Diagnosis and Controversies in Treatment. Breast Care (Basel). 2010: 5; 170-173. [Crossref], [Google Scholar], [PubMed]
- Bednar F, Scheiman JM, McKenna BJ. Breast cancer metastases to the pancreas. J Gastrointest Surg. 2013: 17; 1826-1831. [Crossref], [Google Scholar], [PubMed]
- Molino C, Mocerino C, Braucci A. Pancreatic solitary and synchronous metastasis from breast cancer: a case report and systematic review of controversies in diagnosis and treatment. World J Surg Oncol. 2014; 12. [Crossref], [Google Scholar], [PubMed]
- Nakeeb A, Lillemoe KD, Cameron JL. The role of pancreaticoduodenectomy for locally recurrent or metastatic carcinoma to the periampullary region. J Am Coll Surg. 1995; 180 188-192. [Google Scholar], [PubMed]
- Le Borgne J, Partensky C, Glemain P. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology. 2000; 47: 540-544. [Google Scholar], [PubMed]
- Niedergethmann M, Richter A, Wendl K. Rare indications for a Kausch-Whipple procedure. Eur J Surg. 2001; 167: 115-119. [Crossref], [Google Scholar], [PubMed]
- Moussa A, Mitry E, Hammel P. Pancreatic metastases: a multicentric study of 22 patients. Gastroenterol Clin Biol 2004; 28: 872-876. [Crossref], [Google Scholar], [PubMed]



