Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 9
Does the mean heart dose remain a valid parameter for assessing early cardiac toxicity following radiotherapy for left-sided breast cancer?
Nezha Tawfiq1,2*, Salma Guendaoui1, Meriem Tantaoui1,3, Karima Bendahhou4, Ghita Hatim1, Tarik Chekrine1, Meryem Belhouari1, Mouna Boughafour1, Zineb Bouchbika1, Nadia Benchakroun1, Hassan Jouhadi1, Souha Sahraoui1, Salah Hayar5, Hamza Karmouchi5 and Rachida Habbal2,52Cellular and Molecular Pathology Laboratory, faculty of medicine and pharmacy/ Hassan II University / Casablanca BP 7955, Morocco
3Subatomic Research and Applications Team, Laboratory of the Physics of Condensed Matter (LPMC-ERSA), Faculty of Sciences Ben M’Sick, Hassan II Univers, Morocco
4Department of Epidemiology and Public Health, Ibn Rochd Hospital Casablanca Cancer Registry, faculty of medicine and pharmacy/ Hassan II University, C, Morocco
5Cardio-oncology unit, cardiology department / faculty of medicine and pharmacy/ Hassan II University/ Ibn Rochd University Hospital, Casablanca BP 795, Morocco
Nezha Tawfiq, Mohammed VI Oncology Center/ faculty of medicine and pharmacy/ Hassan II University/ Ibn Rochd University Hospital, Casablanca BP 7955, Morocco, Email: tawfiq2801@gmail.com
Received: 30-Jul-2025, Manuscript No. OAR-25-168387; , Pre QC No. OAR-25-168387 (PQ); Editor assigned: 20-Aug-2025, Pre QC No. OAR-25-168387 (PQ); Reviewed: 08-Sep-2025, QC No. OAR-25-168387; Revised: 22-Sep-2025, Manuscript No. OAR-25-168387 (R); Published: 30-Sep-2025
Abstract
Context: Adjuvant radiotherapy for left-sided breast cancer improves locoregional control and overall survival. However, irradiation of the cardiac structures increases the risk of radiation-induced cardiotoxicity. The mean heart dose (MHD) is commonly used to estimate this risk, but it does not reflect heterogeneous dose distribution within critical substructures, such as the left ventricle (LV) and left anterior descending artery (LAD). Purpose This study aimed to analyze the dose distribution within cardiac substructures and evaluate the reliability of mean heart dose (MHD) in predicting early radiation-induced myocardial dysfunction. Methods: Fifty patients with left-sided breast cancer who underwent three-dimensional conformal radiotherapy (3D-CRT) at Ibn Rochd University Hospital between 2020 and 2022 were analyzed. The target volumes and cardiac substructures were delineated. The dosimetric parameters of the heart, left ventricle (LV), left anterior descending artery (LAD), and other coronary arteries were statistically evaluated. Echocardiographic assessments, including speckle-tracking imaging, were performed before and after radiotherapy, to detect early myocardial alterations. Results: The mean heart dose (MHD) was 4.9 ± 0.8 Gy, while the left ventricle (LV) received a mean dose of 7.7 ± 2.1 Gy. The left anterior descending artery (LAD) was the most exposed structure with a mean dose of 25.9 ± 3.4 Gy. Pearson correlation analysis revealed a strong association between MHD and the mean LV dose (r = 0.742, p < 0.001), but a weaker correlation with the mean LAD dose (r = 0.460, p < 0.001). Regression analysis demonstrated that MHD had a low predictive value for LAD exposure (R² = 0.21) and moderate predictive value for LV dose (R² = 0.55). Echocardiographic assessment revealed segmental strain impairment in 23% of the patients within the LAD-perfused myocardial territory. The mean LAD dose was significantly higher in the patients with segmental strain abnormalities (30.19 Gy vs. 21 Gy, p = 0.004). In contrast, no significant association was found between MHD and strain abnormalities (p = 0.504). Conclusion: This study demonstrated that the mean heart dose (MHD) is insufficient to accurately assess cardiovascular risk in left-sided breast radiotherapy because it underestimates the actual exposure of the left ventricle and left anterior descending artery (LAD). Therefore, these substructures should be considered distinct organs at risk, and specific dosimetric constraints should be applied to better prevent radiation-induced cardiac complications. Moreover, the use of IMRT techniques, respiratory motion management, and early implementation of strain imaging warrants particular attention to optimize the management of high-risk patients.
Keywords
Left-Sided Breast Cancer; External Beam Radiotherapy; Mean Heart Dose; Radiation-Induced Cardiotoxicity; Cardiac Substructures; Cardiac Strain.
INTRODUCTION
Breast cancer is the most common cancer among women worldwide, with an incidence rate of 39.1 per 100,000 in Morocco from 2018 to 2021 and a steadily increasing trend [1]. In 2022, the World Health Organization (WHO) reported approximately 2.3 million new cases and 670,000 deaths attributable to this disease [2]. Adjuvant radiotherapy remains a cornerstone of treatment, significantly improving locoregional control and overall survival [2,3]. However, when the left breast is irradiated, part of the heart is included in the radiation field, leading to an increased risk of long-term cardiac toxicity [5].
The cardiovascular side effects of breast radiotherapy are well-documented. Long-term follow-up studies have shown excess cardiac morbidity and mortality following irradiation of the left breast with an increased prevalence of ischemic heart disease and coronary artery stenosis. Historically, breast radiotherapy has evolved from two-dimensional (2D) techniques, characterized by approximate target volume delineation and limited organ-at-risk protection, to three-dimensional (3D) techniques, which provide better visualization and sparing of the cardiac structures. Recent advances in respiratory gating, intensity-modulated radiotherapy (IMRT), and volumetric modulated arc therapy (VMAT) have further reduced cardiac exposure, potentially lowering the risk of cardiovascular complications in patients treated for left-sided breast cancer[6], [7], [8].
Traditionally, mean heart dose (MHD) has been used as a reference dosimetric parameter. However, this approach fails to capture the heterogeneity of the dose distribution within cardiac substructures, particularly the left anterior descending artery (LAD) and left ventricle (LV) [9]. Recent data suggest that MHD underestimates the exposure of critical cardiac regions, whereas the doses delivered to these substructures are more predictive of late-onset complications [10], [11].
The objective of our study was to analyze the individual radiation dose distribution across cardiac substructures and determine whether MHD remains a reliable dosimetric parameter for assessing substructure exposure and detecting early forms of radiation-induced cardiac complications, especially in asymptomatic high-risk patients, given the current lack of robust evidence regarding early cardiotoxic effects following left-sided breast radiotherapy.
MATERIALS AND METHODS
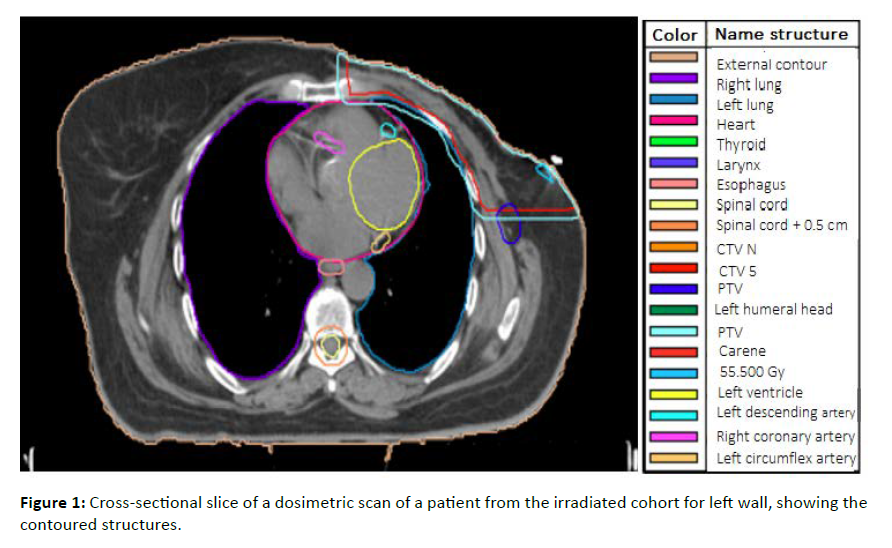
This cross-sectional study included 50 female patients diagnosed with left breast cancer who underwent adjuvant radiotherapy at Ibn Rochd University Hospital in Casablanca between January 2020 and June 2022. Irradiation targeted the entire breast or thoracic wall, with or without inclusion of regional lymphatic areas, but without systematic irradiation of the internal mammary chain (IMC). This decision was based on the absence of clinical indications for IMC irradiation. The acquisition of dosimetric scans was performed without contrast injection and with free-breathing using slices of 3 mm thickness. Target volumes were delineated according to the Radiation Therapy Oncology Group (RTOG) [10]. The delineation of the cardiac substructures was manually performed post hoc according to the cardiac contouring atlas of Duanes et al. [11]. The heart cavities and coronary arteries were delineated and analyzed (the whole heart, left ventricle (LV), coronary arteries (LAD], left circumflex artery (LCx), and right coronary artery (RCA)), as shown in [Figure 1].
Figure 1: Cross-sectional slice of a dosimetric scan of a patient from the irradiated cohort for left wall, showing the contoured structures.
All treatments were performed using three-dimensional conformal radiotherapy (3D-CRT). The patients received hypofractionated radiotherapy at a dose of 40 Gy in 15 fractions (2.67 Gy over 3 weeks), with an additional boost of 13.35 Gy to the tumor bed, if necessary. All included patients received a mean heart dose (MHD) ≥ 5 Gy. The selection of this moderately high-risk population, based on the recommendations of the European Society of Cardiology (ESC) [12], aimed to follow these patients to detect early subclinical anomalies. The thoracic morphology of this population did not allow for acceptable coverage (>95% of the prescribed dose) of the target volume while maintaining an MHD of < 5 Gy.
Ballistics and Treatment Planning
Treatment planning was performed using the Monaco® planning system (version 5.1) with the conformal radiotherapy (3D-CRT) technique. The adopted technique relied on two opposing tangential beams of 6 MV or a mix of 6 MV and 18 MV energies adjusted to encompass the tumor volume while limiting the dose to adjacent structures, particularly the heart and ipsilateral lungs. To improve dose homogeneity, physical compensators or Intensity-Modulated Radiation Therapy (IMRT) were applied based on the patient’s anatomy and dosimetric constraints. Field-in-field techniques were also used to mitigate overdosage areas. For patients requiring lymph node irradiation, a supraclavicular field was added using a direct anterior beam, with optimization of the incidence angle to reduce irradiation of the spinal cord and esophagus. Our results were compared with dose constraints according to the German Society of Radiation Oncology (DEGRO) [13] and Quantitative Analyses of Normal Tissue Effects in the Clinic (Quantec) [14] [Table 1]. Dose constraints are necessary and applicable equally, whether in a 50 Gy scheme in 25 fractions or a hypofractionated scheme, such as 40 Gy in 15 fractions. Maintaining appropriate dose limits is essential for patient safety and minimizing the risk of long-term side effects, as indicated by the overall results of clinical trials [15].
| Structure | Dose constraint |
|---|---|
| D max < 40Gy | |
| Heart | V20 ≤ 10% |
| V40 ≤ 5% | |
| D mean <3 Gy | |
| Left Ventricle | V5 < 17% |
| V23 <5% | |
| LAD | D mean <10Gy |
| Structure | Dmax < 20Gy |
Table 1: Dose constraint to heart structures [14], [16], [17].
Positioning and anatomical alignment were validated using daily controlled imaging to ensure optimal treatment reproducibility.
Statistical Analysis
Statistical analysis was performed using parametric tests with SPSS version 28 to assess the differences and relationships between dosimetric measurements. The comparison of mean values across different dosimetric parameters was conducted using the Student’s t-test, with a significance threshold set at p < 0.05, indicating a statistically significant difference. Correlation between variables was assessed by calculating the Pearson’s correlation coefficient (r), considering a strong correlation when r > 0.7. Finally, linear regression analysis was conducted to examine the relationship between the studied parameters, and the model’s goodness of fit was evaluated using the coefficient of determination (R²). An R² value below 0.7 was considered insufficient to establish a reliable prediction.
Cardiac Imaging and Myocardial Functional Assessment
The cardiac function of patients included in the study was evaluated using a systematic echocardiographic approach. Transthoracic echocardiography was performed in 43 patients by a cardiologist at Ibn Rochd University Hospital both before and after radiotherapy to detect potential subclinical cardiac lesions. This examination allowed for the assessment of the left ventricular ejection fraction (LVEF), which was required to be normal (LVEF > 50%) in all patients. Early myocardial dysfunction was further evaluated using an advanced cardiac imaging technique known as strain echocardiography (speckle tracking). This method enabled the analysis of myocardial contractility post-radiotherapy by determining global longitudinal strain (GLS) and segmental strain. A GLS value less than or equal to -18% was considered abnormal, indicating potential myocardial dysfunction. Strain measurements were conducted in accordance with the recommendations of the American Society of Echocardiography [13], using a 16-segment left ventricular analysis model.
RESULTS
Population Characteristics
Table 2, groups together all the characteristics of the study population, including medical antecedents, breast cancer status and the various therapeutic methods used.
| Characteristics | All (n=50) |
|---|---|
| Medical History | |
| Diabetes | 6 (12%) |
| Hypertension | 4 (8%) |
| Peripheral vascular disease | 6 (12%) |
| Depression | 3 (6%) |
| Cancer Stage | |
| I | 2 (04%) |
| II | 17 (34%) |
| III | 31 (62%) |
| Irradiated Volumes | |
| Breast + Boost | 22 (44%) |
| Chest wall | 28 (56%) |
| Chemotherapy | |
| Yes | 47 (94%) |
| No | 3 (06%) |
| Anthracycline-based Chemotherapy | |
| Yes | 44 (88%) |
| No | 6 (12%) |
| Trastuzumab | |
| Yes | 11 (22%) |
| No | 39 (78%) |
| Anthracycline + Trastuzumab Combination | 11 (22%) |
| Hormone Therapy | |
| Yes | 32 (64%) |
| No | 18 (36%) |
| Time Interval Between End of Radiotherapy and Last Follow-up Consultation | 16.98 mois (7-24) |
Table 2: Baseline characteristics of the study population.
Dosimetric and Statistical Analysis
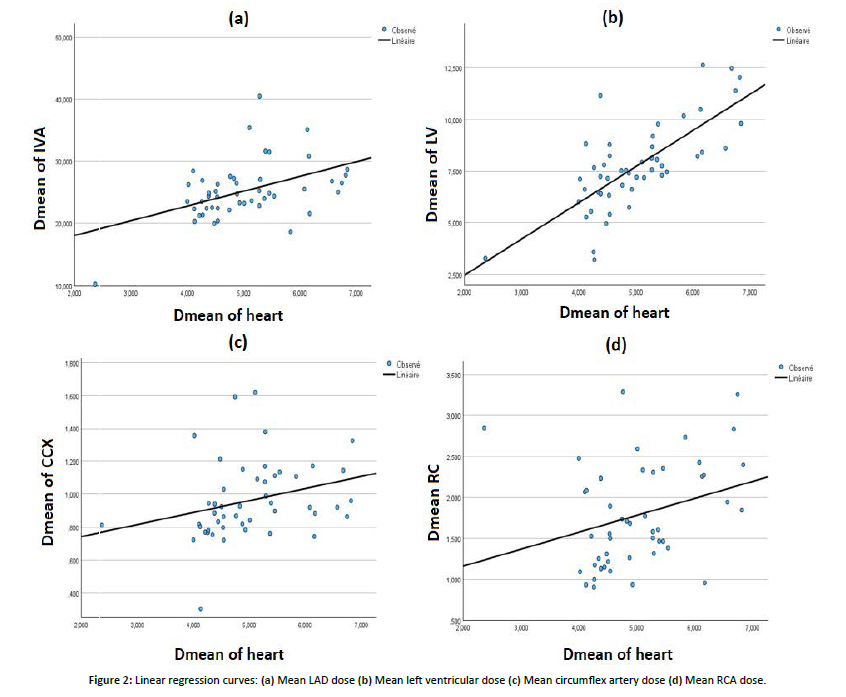
The distribution of doses absorbed by cardiac structures during a hypofractionated radiotherapy protocol and their equivalent in EQD2 highlighted dose variations among the different cardiac substructures. The mean dose received by the whole heart was 4.9 ± 0.8 Gy (5.7 ± 0.7 Gy in EQD2), with a maximum dose reaching 42.8 ± 4.3 Gy (49.3 ± 5.3 Gy in EQD2), indicating a moderate average exposure but a relatively high maximum dose. The left ventricle (LV) received a mean dose of 7.7 ± 2.1 Gy (8.7 ± 2.3 Gy in EQD2), with a maximum dose of 41.3 ± 3.5 Gy (47 ± 4 Gy in EQD2). The irradiated volume doses were significant (V5 = 28.3 ± 8.5% and V23 = 12.4 ± 5.4%). The left anterior descending artery (LAD) was particularly exposed, receiving a mean dose of 25.9 ± 3.4 Gy (28.8 ± 4.8 Gy in EQD2) and a maximum dose of 41.9 ± 4 Gy (48.5 ± 5 Gy in EQD2), suggesting an increased risk of vascular toxicity. In contrast, the left circumflex artery and right coronary artery (RCA) received lower mean doses (1 ± 0.1 Gy and 1.7 ± 0.6 Gy, respectively), although maximum doses for the RCA could reach up to 19.3 ± 13.3 Gy. No statistically significant differences were found between the mean doses to the LV, LAD, and left circumflex artery when comparing breast and chest wall irradiation (p > 0.05), except for the RCA, for which a significant difference was observed (p = 0.037). The Pearson correlation coefficients between the mean heart dose (MHD) and mean doses to the cardiac substructures were all statistically significant. The strongest correlation with MHD was observed for the mean dose to the LV (r = 0.742, p < 0.001). A moderate positive correlation was also noted between MHD and the mean LAD dose (r = 0.460, p < 0.001). [Figure 2], shows that for each 1 Gy increase in MHD, the LAD mean dose increased by an average of 2.37 Gy, while the LV mean dose increased by 1.7 Gy. The linear regression curve showed an R² value of 0.21 for the relationship between MHD and LAD mean dose, indicating that only 21% of the variance in LAD mean dose is explained by variations in MHD. Therefore, MHD has a low predictive value for the mean LAD dose. For the relationship between MHD and LV mean dose, the coefficient of determination (R² = 0.55) indicates that 55% of the variance in LV mean dose is explained by MHD, suggesting that MHD has a low-to-moderate predictive value for LV exposure. Regression curves for the left circumflex artery and RCA showed very weak correlations (R² = 0.08).
Figure 2: Linear regression curves: (a) Mean LAD dose (b) Mean left ventricular dose (c) Mean circumflex artery dose (d) Mean RCA dose.
Cardiac Evaluation
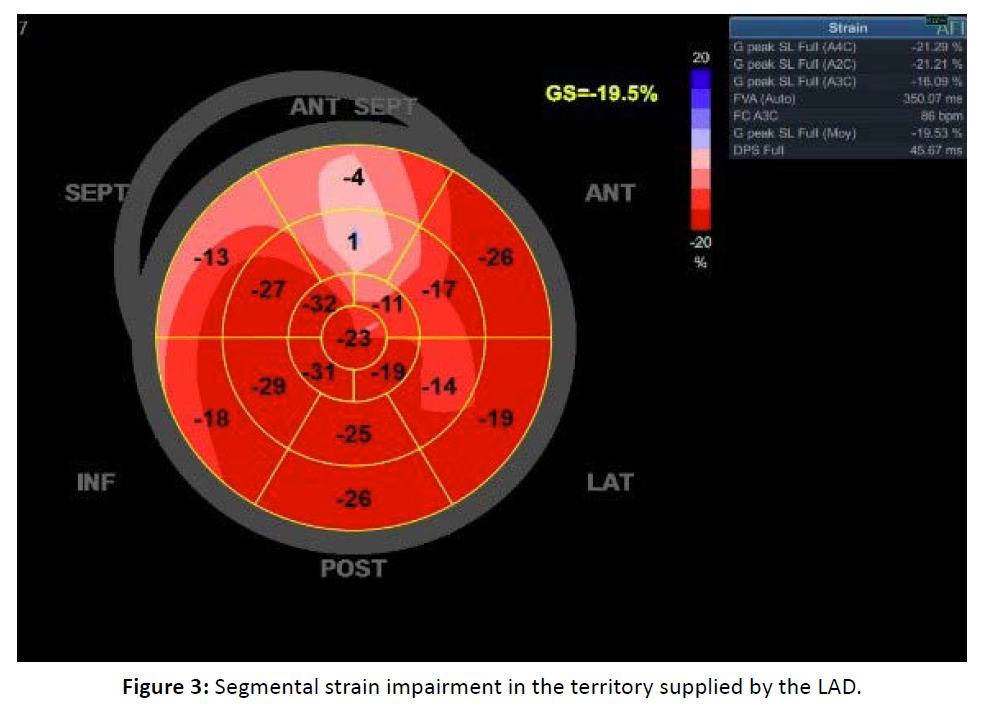
A reduction in global strain was observed in 4 patients (Table 3). Segmental strain was impaired in 23% of patients in the territory supplied by the LAD (Figure 3). A statistically significant association was found between impaired segmental strain in the LAD territory and the mean LAD dose, which was 30.19 Gy in the altered strain group versus 21 Gy in patients with normal segmental strain (p = 0.004). However, no significant association was observed with the mean left ventricular dose (8.24 Gy in patients with altered strain vs. 7.46 Gy in those with normal segmental strain; p = 0.504), nor with the mean heart dose (5.29 Gy vs. 7.46 Gy with p = 0.504).
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 61 | 34 | 52 | 61 |
| History | Hypertension , diabetes | - | - | Hypertension |
| Stage | III | II | II | III |
| Anthracyclines | Yes | Yes | Yes | Yes |
| Trastuzumab | No | No | No | No |
| Dmean of heart (Gy) | 6.4 | 6.3 | 6 | 5.7 |
| Dmean of LV (Gy) | 13 | 12.8 | 9.9 | 8.2 |
| Dmean of LAD (Gy) | 30.3 | 32.4 | 33.6 | 26.6 |
| Global strain | -17 | -15.1 | -16.3 | -16.8 |
| LVEF (%) | 56 | 58 | 57 | 56 |
Table 3: Characteristics of patients with impaired global longitudinal strain.
Figure 3: Segmental strain impairment in the territory supplied by the LAD.
DISCUSSION
This study represents the first Moroccan analysis focusing on dosimetric evaluation of cardiac substructures and early exploration of myocardial dysfunction after left breast radiotherapy. The results are particularly significant as they were obtained in a real clinical context using a cohort of 50 patients treated at a national reference center.
In this study, analysis of cardiac substructures highlights preferential exposure of the left ventricle and left anterior descending artery (LAD). The left ventricle receives a mean dose of 7.7 ± 2.1 Gy (8.7 ± 2.3 Gy in EQD2), with a V5 of 28.3 ± 8.5% and a V23 of 12.4 ± 5.4%, indicating a substantial irradiated volume. The LAD is the most exposed structure, with a mean dose of 25.9 ± 3.4 Gy (28.8 ± 4.8 Gy in EQD2) and a maximum dose of 41.9 ± 4 Gy (48.5 ± 5 Gy in EQD2). These values significantly exceeded the recommended thresholds. Our statistical analyses showed a significant correlation between the mean heart dose (MHD) and mean doses to the cardiac substructures, particularly the left ventricle (r = 0.74, p < 0.001) and LAD (r = 0.46, p < 0.001). However, MHD appears to be only a partial indicator of exposure to these structures, with a low predictive value for the LAD (R² = 0.21) and a moderate predictive value for the left ventricle (R² = 0.55).
In other words, the data highlight substantial and often underappreciated exposure of the LAD and left ventricle, as well as a significant correlation between the mean dose to the LAD and impaired segmental myocardial strain, suggesting a potential functional impact. These results emphasize the need for a more individualized approach for cardiovascular risk assessment and confirm the limitations of using MHD as the sole indicator of cardiovascular risk. Our observations are consistent with those of the previous studies. As seen in the BACCARAT study by Jacob et al. [5], the correlation between MHD and doses to cardiac substructures was weak for the LAD (R² = 0.21) and moderate for the left ventricle (R² = 0.55), supporting the case for individualized evaluation. Functionally, our study concurs with the work of Erven et al. [18], who observed impaired segmental strain in the LAD territory without significant changes in other myocardial regions. The significance of the relationship between the mean dose to the LAD and segmental strain impairment (p = 0.004) observed here is also consistent with the findings from BACCARAT, where LAD exposure > 19.9 Gy was associated with subclinical dysfunction in the corresponding segment.
In contrast to the study by Naimi et al. [19], our study did not find a statistically significant difference in LAD doses between whole-breast and wall irradiation (25.01 Gy vs. 25.33 Gy; p = 0.129). This discrepancy may be explained by differences in the beam geometry, contouring, or sampling methods. Furthermore, the measured values in our cohort far exceeded the thresholds proposed by DEGRO and Abraham et al. [13], [20], which can be attributed to the absence of respiratory gating and advanced techniques such as IMRT or VMAT in our institution.
This study has several limitations. First, the retrospective nature of the study limits causal interpretation. Second, the concomitant use of cardiotoxic chemotherapy (anthracyclines and trastuzumab) was a major confounding factor in the analysis of myocardial function. Third, unenhanced dosimetric scanners may induce underdetection of fine coronary structures. Finally, on a methodological level, it is important to note the difficulty in comparing the initial myocardial strain results with later controls, given the inter-observer and inter-machine variability, which is a crucial factor to consider in future studies.
Ultimately, our results call for the implementation of optimization strategies to reduce cardiac risk. Advanced radiotherapy techniques can further reduce cardiac exposure. The application of intensity-modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) combined with deep inspiration breath-hold (DIBH) or surface-guided radiation therapy (SGRT) allows for better dose conformation and greater sparing of the heart than conventional 3D tangents [21]. Although these modern approaches require a specific dosimetric evaluation of their impact [22], numerous studies have demonstrated significant reductions in cardiac doses. Additionally, close cardiological monitoring of high-risk patients is essential. Systematic integration of myocardial strain measurement by echocardiography at regular intervals following radiotherapy could allow for early detection of subclinical degradation and enable the initiation of cardioprotective measures accordingly [23]. Furthermore, improving the visualization of coronary arteries during planning would be beneficial, and the fusion of simulation CT with coronary angiography (coro-CT) is a promising avenue to better identify coronary paths and refine LAD protection. Finally, the development of automatic delineation tools based on artificial intelligence (AI) can standardize and accelerate the delineation of cardiac substructure, improve dosimetric accuracy, and prevent cardiotoxicity.
CONCLUSION
This study highlights the limitations of the mean cardiac dose (MHD) as a single indicator of cardiovascular risk in left breast radiotherapy. Indeed, our results clearly show that the left ventricle and left anterior descending artery (LAD) can receive significantly high doses, often underestimated when referring only to MHD. Thus, it is essential to consider these cardiac substructures as specific organs at risk during the dosimetric optimization process to improve the risk assessment of radiation-induced cardiotoxicity. This is possible because of the development of artificial intelligence in the delineation of these substructures. In this context, the application of specific dosimetric constraints to these substructures could allow a better assessment of the benefit-risk balance, and thus optimize primary prevention strategies for cardiotoxicity. Prospective studies could evaluate the impact of IMRT in reducing the dose to these substructures. Simultaneously, monitoring a patient’s cardiovascular function will allow for early detection of strain impairment. These patients can receive preventive care to preserve cardiac function.
Statements
Our study was cross-sectional study which did not include an experimental arm
The authors have no conflicts of interest to declare
This study was not supported by any sponsors or funding.
This work was carried out in collaboration with all the authors. All the authors have read and approved the final manuscript.
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
REFERENCES
- Registre des cancers du Grand Casablanca | Recherché en Cancerology-IRC. Consulté le: 10 avril 2025.
- Masson E. Cancer du sein et cardiotoxicité des traitementsâ¯: prévenir, dépister et structurer le suivi cardio-gynécologique. EM-Consulte. Consulté le. 2025. [Google Scholar]
- HUIC Khalifa. InfoSanté: Octobre rose à l’HCK, ensemble pour vaincre de cancer du. Consulté le: 16 mars 2025.
- Taylor C. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol. 2017; 35: 1641â [Crossref] [Google Scholar] [PubMed]
- Jacob S. Limites de la dose moyenne au coeur dans l’evaluation de l’exposition du ventricule gauche et des arteres coronaires au cours d’une radiotherapie pour un cancer du sein: evaluation dosimetrique a l’echelle individuelle (etude baccarat). juin 2019; 14: 29. [Crossref] [Google Scholar] [PubMed]
- Cardiotoxicity: Heart Damage from Cancer Treatment », Cleveland Clinic. Consulté le: 16 mars 2025.
- Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging (PubMed). Consulté le: 16 mars 2025.
- Lang RM. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1-39.e14. [Crossref] [Google Scholar] [PubMed]
- Bowles EJA. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012; 104: 1293â [Crossref] [Google Scholar] [PubMed]
- Ewer MS. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005; 23: 7820â [Crossref] [Google Scholar] [PubMed]
- 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) | European Heart.
- Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO) – PubMed.
- Beaton L. Cardiac death after breast radiotherapy and the QUANTEC cardiac guidelines. Clin Transl Radiat Oncol. 2019; 19: 39â [Crossref] [Google Scholar] [PubMed]
- Haviland JS. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypo fractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013; 14: 1086â [Crossref] [Google Scholar] [PubMed]
- Piroth MD. Heart toxicity from breast cancer radiotherapy. Strahlenther Onkol. 2019; 195: 1â [Crossref] [Google Scholar] [PubMed]
- Erven K. Subclinical Cardiotoxicity Detected by Strain Rate Imaging up to 14 months After Breast Radiation Therapy. International Journal of Radiation Oncology Biology Physics. 2013; 85: 1172â [Crossref] [Google Scholar] [PubMed]
- Naimi Z. Cardiac substructures exposure in left-sided breast cancer radiotherapy: Is the mean heart dose a reliable predictor of cardiac toxicity. Cancer Radiother. 2021; 25: 229â [Crossref] [Google Scholar] [PubMed]
- Abraham A. Is Radiation-Induced Cardiac Toxicity Reversible? Prospective Evaluation of Patients With Breast Cancer Enrolled in a Phase 3 Randomized Controlled Trial. Int J Radiat Oncol Biol Phys. 2022; 113: 125â [Crossref] [Google Scholar] [PubMed]
- Rudat V, Zhao R, Wang B, Zhang L, Shi Y. Impact of deep inspiration breath hold, surface-guided radiotherapy, and daily CBCT on the organs at risk in breast cancer radiotherapy. Sci Rep. 2024; 14: 27814. [Google Scholar]
- Lu Yet. Cardiorespiratory dose comparison among six radiotherapy regimens for patients with left-sided breast cancer. Sci Rep. 2023; 13: 13339. [Crossref] [Google Scholar] [PubMed]
- Vennarini S. Visualisation of the left anterior descending coronary artery on CT images used for breast radiotherapy planning. Br J Radiol. 2013; 86: 20120643. [Crossref] [Google Scholar] [PubMed]
- Wilson J, Hua CJ, Aziminia N, Manisty C. Imaging of the Acute and Chronic Cardiovascular Complications of Radiation Therapy. Circ Cardiovasc Imaging. 2025; 18: e017454. [Crossref] [Google Scholar] [PubMed]
- Walls GM. Validation of an established deep learning auto-segmentation tool for cardiac substructures in 4D radiotherapy planning scans. Physics and Imaging in Radiation Oncology. 2022; 23: 118â [Crossref] [Google Scholar] [PubMed]