Research - Onkologia i Radioterapia ( 2021) Volume 15, Issue 5
The effect of phlebotomy (Fasd) on carpal tunnel syndrome; a randomized clinical trial
Leila Hashemi Chelavi1, Reza Ilkhani1*, Mohadeseh Azadvari2, Hoorieh Mohammadi Kenari3 and Gholamreza Kordafshari42Department of Physical Medicine and Rehabilitation, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
3Schools of Traditional Medicine, Iran University of Medical Sciences, Tehran, Iran
4Department of Traditional Medicine, School of Traditional Medicine, Tehran University of Medical Sciences, Tehran, Iran
Reza Ilkhani, Department of Traditional Medicine, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Email: ilkhanir@sbmu.ac.ir
Received: 10-Apr-2021 Accepted: 27-Apr-2021 Published: 14-May-2021
Abstract
Background and Purpose: Phlebotomy has been broadly used as an assured and economical technique to tackle soft tissue lesions in ITM (Iranian Traditional Medicine), most Asian countries, Central Europe, and some parts of the US. In this study, phlebotomy was used for the treatment of Carpal Tunnel Syndrome (CTS) that is widely recognized as the most common neuropathy. This study aimed to investigate the effects of phlebotomy on the clinical and diagnostic findings of patients with CTS. Methods: This is a randomized clinical trial. For this analysis, an entire of 70 hands with CTS were studied, aged 20-60, and of every gender. The participants were divided into 2 groups: Control and Intervention. Within the control group, 35 were treated with a routine night splint for 3 months, and 35 patients in the intervention group, were received Fasd (Phlebotomy) of Osailem vein in addition to the routine night splint. The variables of which we evaluated were: pain, symptom severity, functional status, distal median nerve sensory latency, and distal median nerve motor latency. The pain has been evaluating by VAS Scale while the severity of symptoms and functional status of patients were evaluated by the Boston questionnaire, and the distal and motor nerve latency were evaluated through the diagnostic NCV test. Results: The results of the study demonstrated an incontestable improvement in symptom severity (P=0/0001) and functional status [Significant treatment effects for the Boston CTS- score (P=∙0007)]. Additionally a serious decrease in Median nerve entrapment with NCV (p=0∙015) of the intervention group in comparison with the control one. Pain severity of CTS symptoms (VAS), was also reduced significantly from 61.5 ± 20∙5 to18.9 ± 15∙7mm after 3 months in the phlebotomy group and from 67∙1 ± 20∙2 to 52∙7 ± 21∙8mm in the control group [group difference –33.8mm (P<∙001). Conclusions: The results showed that the incorporation of phlebotomy (Fasd) treatment in an exceedingly routine therapy program would reduce the pain and symptom severity, improve the functional status of patients, and distal sensory and motor disturbance of the median nerve. Therefore, it is advised that phlebotomy (Fasd), as a convenient and low‐cost complementary medicine technique could be employed within the treatment of CTS.
Keywords
carpal tunnel syndrome, Iranian traditional medicine, phlebotomy, Fasd, randomized clinical trial
Introduction
Carpal Tunnel Syndrome (CTS) is a common disorder with an estimated prevalence of 3.8% (clinically and electro physiologically confirmed) in the general population [1]. Women are more frequently affected than men [2]. CTS causes significant morbidity [3] and has, in addition to its potentially debilitating physical aspects, a negative financial impact resulting from time lost from work and increased medical expenditures [4]. Classic symptoms of CTS include numbness, tingling, burning, and pain in at least 2 of the 3 digits supplied by the median nerve (i.e., thumb, index finger, and middle finger). These symptoms are highly prevalent (14.4%) in the general population [5].
CTS results from entrapment of the median nerve in the carpal tunnel part of the wrist [2]. Pathologically the consequence of non-inflammatory fibrosis of the sub synovial connective tissue surrounding the flexor tendons. Biochemical studies of surgical specimens suggest that a variety of regulatory molecules and bifid median nerve may induce fibrous and vascular proliferation, possibly as a response to mechanical stress [6]. But CTS is also related to systemic factors such as metabolic and endocrine disorders, obesity, and amyloid degeneration [7]. Most cases of CTS have no readily identifiable cause (idiopathic CTS). Whether more proximal disorders, i.e., cervical radiculopathies or musculoskeletal pain syndromes affecting referred, or segmentally related, zones, can predispose to injury at sites distal to their lesions and thus be involved in the pathogenesis of CTS as proposed by the double crush hypothesis remains controversial [8].
Standard treatment of CTS in the past has included wrist splints, oral anti-inflammatory agents, and avoidance of occupational duties, locally injected corticosteroids, and surgery. However, symptomatic relief with conservative treatments has been less than satisfactory, and surgical decompression, often considered the definitive solution, yields good results in only 75% of cases [9]. Since the standard treatments for CTS are not fully satisfactory, other conservative methods, including those from traditional and alternative medicine, need to be further evaluated.
Phlebotomy (Fasd) has been broadly used as an assured and economical technique to tackle soft tissue inflammations in ITM (Iranian Traditional Medicine), most Asian countries, Central Europe, and some parts of the US. Many studies in recent years have tried to clarify the efficacy of phlebotomy in a variety of disorders [10]. Some of them have reported the beneficial effects of phlebotomy in clinical investigations. The most-reported positive effect of this technique is pain relief [11]. Although much evidence shows the probable effect of phlebotomy in medical aims, there are too many concerns for it to be applied in society [12]. ITM scholars like Avicenna advised phlebotomy (Fasd) for preventing serious diseases [13]. Phlebotomy (Fasd) is a TPM blood-letting method in which a small incision is made in one of the superficial veins of the body and blood is taken.
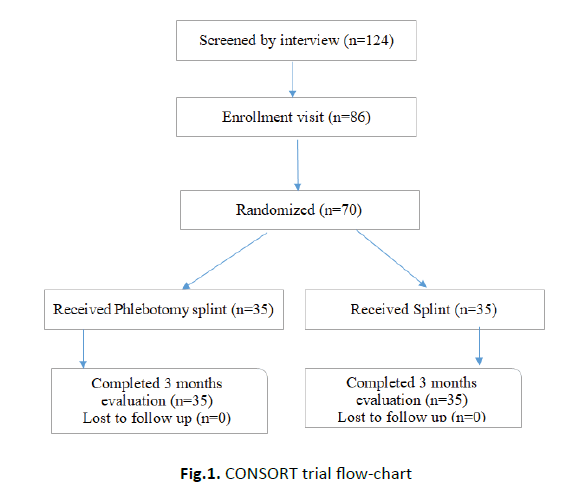
In this study, phlebotomy (Fasd) of Osailem Vein (the dorsal digital vein between the 4th and 5th finger) was used for the treatment of Carpal Tunnel Syndrome (CTS) that is widely recognized as the most common neuropathy. Therefore, we aim to consider the therapeutic effect of phlebotomy (Fasd), which has been using as an effective method for treatment of inflammation and articular pain in Iranian Traditional medicine, in the treatment of CTS (Figure 1).
Figure 1: CONSORT trial flow-chart
The phlebotomy (Fasd) of the dorsal hand segmentally related to the median nerve to treat CTS has been practiced in Iranian Traditional Medicine and is supported by recent research.
In a cross-sectional study, typical alterations of the connective tissue such as painful hardening of the sub cutis, adhesion or swelling of subcutaneous tissue and fascia, and reduced microcirculation in the shoulder triangle, were found to be associated with the severity of CTS symptoms [14]. Since a preliminary clinical trial has shown that phlebotomy (Fasd) of this region has no priority to any other treatments in alleviating the symptoms of CTS [13]. We conducted a randomized clinical trial to evaluate the effectiveness of phlebotomy (Fasd) of a mentioned area of a significant segment of dorsal hand (Osailem area) by comparing it with a control routine treatment in patients with moderate symptomatic CTS.
Methods
This study was designed as a randomized, clinical trial. All study participants gave their informed consent. The study protocol was reviewed and approved by the Ethics Committee of, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences. Patients were screened and recruited between November to June 2017. Treatments and follow-ups of the patients were completed by October 2017.
Potential participants were screened for eligibility by interview and filling the Boston questionnaire and, and eligible candidates were scheduled for the NCV test. A Rehab Specialist Professor performed the candidates’ physical examinations and NCV test. Thereafter, each participant was randomly assigned to the phlebotomy (Fasd) plus night splint or the routine night splint therapy group, and the respective treatment started. All participants were followed on days 30 and 90 after the allocated treatment by interview (Boston Questionnaire, VAS scale), and final NCV performed after 3 months of treatment for all study participants.
Patients of both sexes were eligible if they were between 20 years and 60 years old and suffered from moderate CTS as confirmed by NCV and Boston questionnaire. Only patients who had any of the systematic underlying factors such as DM, hyperthyroidism, RA, Lupus, patients participating in another study, patients with a past medical history of previous surgery for CTS, or had had intra-articular injections within the previous 3 months, were excluded.
Also, Patients did not consider eligible to take part in the study if they were receiving anticoagulants or had haemophilia, anaemia, polyneuropathy, or a coexisting serious illness. Patients regularly taking NSAIDs or analgesics as rescue medication were not excluded if the mean weekly dosage and type of administration had not been altered during the preceding 3 months (Figure 2).
Figure 2: Total symptom score. Means 6 SD of the total of symptom severity and NCV scores in the Phlebotomy and the Control (splint therapy) groups during the course of the study. P values were calculated from repeated measurement ANCOVA
Randomization
Patients were randomly allocated to the 2 treatments by a non-stratified even-odd- randomization by sequentially patient’s reference numbers containing the treatment assignments. Randomization and the paper files were prepared by the study biostatistician. When a patient fulfilled all enrolment criteria, the study physician enters them in one of the two groups due to his reference number randomly.
Intervention
Protocol for performing phlebotomy (Fasd) was as follows: The skin of dorsal hand (Dorsal metacarpal veins area) between 4th and 5th finger was disinfected; Puncturing of the skin was carried out superficially by using a sterile micro lancet and letting the blood eject from that small incision till stopped, then treated area was bandaged. Each patient was incised only once. Both Intervention and control groups received the routine treatment consisted of applying night splint for all the study duration (3 months).
Outcome measures
The primary outcome measure was the change in total CTS symptom severity from day 0 to 30 as derived from the mean of the patients’ 100 mm Visual Analog Scale (VAS) symptom scores (global pain, tingling, and numbness).
All VAS scores were assessed at the first visit and the end of both the first and 3rd months and recorded. Other secondary outcomes included the symptom severity score of CTS as measured by the Boston questionnaire, all questionnaires were filled in at baseline at the first visit after randomization and in addition at the end of the first and third month. Patients were asked to record any adverse effects of their treatment and their use of oral analgesic medication.
Analysis
The study was powered to detect the changes in both control and intervention groups. Of the individuals initially screened by interview, 70 were invited to be further assessed. Of these, the first 70 that fulfilled all study criteria and agreed to participate in the study were included, 35 being randomly assigned to the phlebotomy (Fasd) group and 35 to the control group. All patients had neurologically confirmed moderate CTS for which they had previously received treatment. The most frequent treatment, a wrist splint, had been applied in all patients in each group (Table 1).
Tab. 1. Severity of carpal tunnel syndrome symptoms assessed with visual analog scale subscales and boston questionnaire in study groups with group differences for change on treatment
| Base Line | At the end of study | Group Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Pain at rest | ||||
| Phlebotomy | 61.5 ± 6 24.9 | 25.2 ± 6 25 | 24.5 (-36.3; -11.5) | < .001 |
| Routine Night Splint Therapy | 58.6 ± -25.1 | 47± 27.7 | ||
| Numbness | ||||
| Phlebotomy | 61.1 ± 6 28 | 21.4 ± 24.6 | -28.8 (-42.5; -15.1) | < .001 |
| Routine Night Splint Therapy | 72.9 ± 6 22.6 | 54.4 ± 25.5 | ||
| Tingling | ||||
| Phlebotomy | 61.3 ± 6 22.4 | 24.3 ± 23.7 | -25.2 (-37.8; -12.6) | < .001 |
| Routine Night Splint Therapy | 70. 6 ± 20.5 | 52.9 ± 25 | ||
| Pain movement | ||||
| Phlebotomy | 64.6 ± 23 | 29.2 ± 28.2 | -32.4 (-45.5; -19.3) | < .001 |
| Routine Night Splint Therapy | 60.1 ± 6 28.1 | 60.5 ± 28.8 | ||
| Boston CTS Score Symptom severity | ||||
| Phlebotomy | 3.1 ± 6 .6 | 2.4 ± 6 .8 | -0.6 (-0.9; -0.2) | < .002 |
| Routine Night Splint Therapy | 3.2 ± 6 .8 | 3.0 ± 6 .7 | ||
| Functional status | ||||
| Phlebotomy | 2.5 ± 6 .8 | 1.9 ± 6 .6 | -0.6 (-0.8; -0.3) | < .001 |
| Routine Night Splint Therapy | 2.6 ± 6 .8 | 2.6 ± 6 .8 | ||
NOTE: Mean values 6 SD and estimated group difference (95% CI)
Results
Outcome Measures Phlebotomy (Fasd) therapy was more beneficial than splint, according to the primary outcome measure, change in the total symptom score after day 30,90 the average (6 SD) symptom score was reduced from 28.57 to 25.57. 22.68 mm decrease at day 30 and day 90 in the phlebotomy (Fasd) group with a highly significant between-group difference of -24.5 mm [CI: -36.1; -12.9; p<.001 (repeated measurement]. Comparably significant group differences favouring the phlebotomy (Fasd) therapy were found with all 3 subscales of the sum score, the CTS VAS pain scales, and the Boston CTS questionnaire and NCV. Disability in daily life as assessed by the Boston questionnaire score was rapidly allayed with phlebotomy (Fasd) therapy, resulting in a significant difference between the two groups. Finally, quality of life improved only for the phlebotomy (Fasd) group at day 30 and 90 [mean group difference 0.3 (95% CI 0; 0.3); p=0.040].
The use of oral analgesics was comparable in both groups throughout the study. On average, the participants used rescue medication on fewer than 4% of all patient study days.
A regular minor adverse effect was a hematoma at the site of application of a phlebotomy (Fasd) area. All scarified wounds healed without complication. None of the patients rated the phlebotomy (Fasd) procedure as painful, and all patients in both groups perceived their study treatment as very tolerable.
Discussion
Symptomatic CTS is highly prevalent in all populations [15,16]. Since conservative options for treatment are limited [17]. New therapeutic approaches need to be considered. It has recently been proposed that phlebotomy (Fasd), an Iranian traditional Medicine Method of treatment, may be beneficial in symptomatic CTS when applied to dorsal digital veins between the 4th and 5th digits. Phlebotomy (Fasd) is used to treat pain and inflammation in various different ethno medical systems [18] and a recent randomized study suggested that phlebotomy (Fasd) alleviates low back pain. We designed the present study to further evaluate this method of treatment. In this study, patients with CTS who were treated with phlebotomy (Fasd) of Osailem Vein experienced a highly significant decrease in pain and CTS symptoms and even significant changes in NCV. Moreover, a single treatment improved functional ability (according to the Bostom Questionnaire) and quality of life and reduced associated pain. The observed improvements are most likely attributable to the therapeutic intervention, confirming the results of the recent pilot study [19].
In this randomized clinical trial, the mean pain score at rest decreased by 59%, and the mean symptoms score decreased by 60%. Both scores reflect substantial improvement. Moreover, the magnitude of the phlebotomy (Fasd) intervention was 1.2 points, which is a large and clinically relevant effect. At the outset, the symptom scores of the patients in the control group were slightly higher than those in the phlebotomy (Fasd) group, which may bias the results. But with the exception of pain with motion, the baseline differences were not significant. Since the study was randomized, these differences must have occurred by chance. Higher scores for numbness and tingling in the control group may reflect that this group had a poorer prognosis. Since the higher scores were offset by lower pain-at-rest scores, using the average score as a covariate in the analysis may not have adequately reflected prognosis. We therefore conducted an additional analysis in which the single scores (pain, tingling, and numbness) were used as covariates. This analysis resulted in even larger post treatment group differences, thus corroborating our main results. Severity of Carpal Tunnel Syndrome Symptoms Assessed with Visual Analog Scale bias due to group differences at baseline can be regarded as negligible. The mechanisms have been considered to explain the observed effects. First, the phlebotomy (Fasd) may have been effective due to its direct effect on the inflammation in the wrist area. In addition it may help the decompression in this area as well as reducing the pressure in any anatomically variant veins. Therefore, it remains unclear whether phlebotomy (Fasd) works via its effects on proximal nerve function. Second, nociceptive activation contributes to chronic pain [20] and phlebotomy (Fasd) may alleviate pain by means of antinociceptive effects and by counter irritation. However, at present, it is unclear to what extent phlebotomy (Fasd) induces such mechanisms.
Third, phlebotomy (Fasd) therapy may simply have a powerful placebo effect. In fact, all invasive or no pharmacological treatments may have relevant placebo effects. In a recent randomized trial, a sham device was more effective in relieving pain than a placebo pill. Therefore, the nonspecific and placebo effects of phlebotomy (Fasd) therapy may result from the fact that it is an uncommon procedure. However, this is relevant only if placebos are indeed effective in treating chronic pain syndromes, which remains unproven [21].
Conclusion
This study is limited because it is an open trial. Placebo like and unspecific treatment effects cannot be well controlled and precisely assessed. To date, it has not been possible to blind for complex procedures like phlebotomy (Fasd). Furthermore, since most Persian patients are familiar with phlebotomy (Fasd), they may be able to guess which treatment they received, thus compromising study results. For these reasons we first assessed the effectiveness of phlebotomy (Fasd) in a trial.
Since the effect of the phlebotomy (Fasd) intervention in a population with chronic pain was significant (N=35), it seems unlikely that it can be fully explained by unspecific effects with nonbinding. Furthermore, we assessed outcome expectation in order to approximate the placebo effects. Scores did not indicate that the phlebotomy (Fasd) group had higher expectations, and overall results did not change after adjustment for the confounding effect of outcome expectation. Therefore, although a relevant effect of phlebotomy (Fasd) is very likely an unspecific one, our results indicate that this treatment may also have a specific effect.
To better assess the nonspecific treatment effects of Fasd, a specific Fasd procedure should be developed for future trials.
In clinical practice, phlebotomy (Fasd) is conveniently and easily performed and thus suitable for repetitive treatments. Further studies are needed to assess the long-term value of phlebotomy (Fasd) in the management of CTS. The therapeutic effect of phlebotomy (Fasd) may seem greater because of the control treatment to which it was compared.
The outcome expectation score in the control group suggests these participants expected their treatment to be effective. Future trials should also compare phlebotomy (Fasd) for CTS with other standard treatments such as steroid injections, surgery and etc.
Phlebotomy (Fasd) therapy as applied in this study was safe and very well tolerated. A common minor adverse effect was a local hematoma, but wound healing after phlebotomy (Fasd) was uncomplicated. In conclusion, a single course of phlebotomy (Fasd) of the Osailem vein Change of overall symptom score, functional status, pain severity and a remarkable change in NCV test result (study day 90) and patients’ expectations at baseline. The efficacy of this treatment and its related mechanisms should be further studied in blinded, randomized trials of longer duration using other treatments as controls.
References
- Genova A, Dix O, Saefan A, Thakur M, Hassan A. Carpal tunnel syndrome: a review of literature. Cureus. 2020;12:e7333.
- Geoghegan JM, Clark DI, Bainbridge LC, Smith C, Hubbard R. Risk factors in carpal tunnel syndrome. J Hand Surg Am. 2004;29:315-320.
- Michalsen A, Bock S, Lüdtke R, Rampp T, Baecker M, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
- Park JJ, Choi JG, Son BC. Carpal tunnel syndrome caused by bifid median nerve in association with anomalous course of the flexor digitorum superficialis muscle at the wrist. The Nerve. 2017;3:21-23.
- Jang S, Choi J, Son B. Compression of the median nerve by a lipoma in the distal forearm associated with bilateral carpal tunnel syndromes. The Nerve. 2016;2:84-86.
- Chen L, Chen J, Hu B, Jiang LX. Sonographic findings of the bifid median nerve and persistent median artery in carpal tunnel: a preliminary study in chinese individuals. Clinics (Sao Paulo). 2017;72:358-362.
- Lee S, Cha E, Yang M, Lee J, Lee S, et al. The effects of acupotomy therapy on carpal tunnel syndrome: a report of 4 cases. J Acupunct Res. 2018;35:4-10.
- Juul R, Tarnowski JR, Houe T. Carpal tunnel syndrome caused by vascular nerve impairment treated with open release of the carpal tunnel. Case reports Plast Surg hand Surg. 2015;2:29-30.
- Aboonq MS. Pathophysiology of carpal tunnel syndrome. Neurosciences (Riyadh). 2015;20:4-9.
- Setayesh M, Zargaran A, Sadeghifar AR, Salehi M, Rezaeizadeh H. New candidates for treatment and management of carpal tunnel syndrome based on the Persian Canon of Medicine. Integr Med Res. 2018;7;126-135.
- Kordafshari G, Ardakani MRS, Keshavarz M, Esfahani MM, Nazem E, et al. The role of phlebotomy (Fasd) and wet cupping (Hijamat) to manage dizziness and vertigo from the viewpoint of persian medicine. J Evidence-Based Complement Altern Med. 2017;22:369-373.
- Atyabi AS, Nejatbakhsh F, Kenari HM, Eghbalian F, Ayati MH, et al. Persian medicine non-pharmacological therapies for headache: phlebotomy and wet cupping. J Tradit Chinese Med. 2018;38:457-464.
- Zhang YH. Clinical application of bloodletting method on the face. Zhongguo Zhen Jiu. 2011 ;31:639-641.
- Qiu XH, Xie XK, Liu XN. Clinical observation on pricking blood along meridians combined with electro acupuncture for treatment of prolapse of lumbar intervertebral disc. Zhongguo Zhen Jiu. 2010;30:985-988.
- Atroshi I, Gummesson C, Johnsson R, Ornstein E. Diagnostic properties of nerve conduction tests in population-based carpal tunnel syndrome. BMC Musculoskelet Disord. 2003;4:9.
- Michalsen A, Bock S, Lüdtke R, Rampp T, Baecker M, et al. Effects of traditional cupping therapy in patients with carpal tunnel syndrome: a randomized controlled trial. J Pain. 2009;10:601-608.
- De Kleermaeker FGCM, Boogaarts HD, Meulstee J, Verhagen WIM. Minimal clinically important difference for the Boston Carpal Tunnel Questionnaire: new insights and review of literature. J Hand Surg. 2019;44:283-289.
- Kordafshari G, Ardakani MRS, Keshavarz M, Esfahani MM, Nazem E, et al. The role of phlebotomy ( Fasd) and wet cupping ( Hijamat) to manage dizziness and vertigo from the viewpoint of persian medicine. J Evid Based Complementary Altern Med. 2017;22:369-373.
- Lüdtke R, Albrecht U, Stange R, Uehleke B. Brachialgia paraesthetica nocturna can be relieved by “wet cupping”-results of a randomised pilot study. Complement Ther Med. 2006;14:247-253.
- Kordafshari G, Ardakani MRS, Keshavarz M, Esfahani MM, Nazem E, et al. The role of phlebotomy ( Fasd) and wet cupping ( Hijamat) to manage dizziness and vertigo from the viewpoint of persian medicine. J Evid Based Complementary Altern Med. 2017;22:369-373.
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. The Lancet. 2010;375:686-695.