Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 8
Synthesis and characterization of new 1, 3, 4-thiadiazole derivatives of naproxen as a potential antineoplastic agent
Aamer A. Chaab1* and Mazin N. Mousa22Department of Pharmaceutical Chemistry, College of Pharmacy, University of Basrah, Basrah, Iraq
Aamer A. Chaab, Basrah Health Directorate, Ministry of Health, Basrah, Iraq, Email: aameralchaab90@gmail.com
Received: 16-Jun-2023, Manuscript No. OAR-23-102922; Accepted: 20-Aug-2023, Pre QC No. OAR-23-102922 (PQ); Editor assigned: 28-Jul-2023, Pre QC No. OAR-23-102922 (PQ); Reviewed: 09-Aug-2023, QC No. OAR-23-102922 (Q); Revised: 16-Aug-2023, Manuscript No. OAR-23-102922 (R); Published: 23-Aug-2023
Abstract
Cancer is a dangerous issue affecting the validity of each human community, which interferes with a cellular connection and results in critical gene dysfunction. This disorder is efficient in the cell cycle and causes proliferation abnormality. This study was performed in the synthesis of five derivatives of 1, 3, and 4-thiadiazole associated with Schiff bases. The synthesis was initiated by the reaction of Naproxen with thiosemicarbazide in the presence of Phosphorous Oxychloride (POCl3), which undergoes cyclization reaction upon neutralization with sodium bicarbonate aqueous solution forming 1, 3, 4-thiadiazole. Then it was reacted with different aromatic aldehydes converted to Schiff bases. The chemical structure of newly synthesized compounds was emphasized by using 1H-NMR, 13C-NMR, FT-IR, and C, H, N analysis. The activity of all synthesized compounds by Mtt assay to evaluated comparison with standard drug (Naproxen). It was revealed activity upon cellular inhibition on Mtt. The newly synthesized compounds are successfully prepared depending on FT-IR, 1H-NMR, 13C-NMR, and C, H, N analysis data, and they revealed anti-neoplastic activity compared with standard
Keywords
thiadiazole, antineoplastic, schiff base, mtt assay, naproxen
Introduction
Cancer is a dangerous issue affecting the validity of each human community. Unfortunately, at the tissue level, it is a diverse illness, and this diversity is a significant challenge for its special diagnosis, and treatment effectiveness [1, 2]. It takes place via a series of sequential gene mutations so that such mutations alter cell functions. Chemicals have an evident role in the formation of gene mutations and cancer cells. Smoking contains numerous carcinogenic chemicals that cause lung cancer [3]. Interestingly, the carcinogenic environmental chemicals indirectly or directly impact the cell nucleus and cytoplasm, and resulted in gene disturbances and mutations [4-7].
Generally, cancer interferes with a cellular connection and results in critical gene dysfunction. This disorder is efficient in the cell cycle and causes proliferation abnormality [8, 9]. The proto-oncogenes are accountable for the growth and division of the cells in natural circumstances but get oncogenes through genetic mutation, which is more serious for cell's existence [10]. In addition, the uncontrolled cell division caused by the absence of tumour suppressor genes causes [11].
Thiadiazole is the more prevail, indispensable heterocyclic moiety. It is a five-member heterocyclic moiety (Heterocyclic moiety that has a five-membered ring containing two nitrogen and one sulphur atom). A scaffold that makes a very important structure for many naturally occurring compounds and products of medical importance [12].
Naggar et al. (2019) lately provided design, synthesis, and molecular docking experiments on the anti-cancer activity of 5-(3,5-dinitrophenyl)-1,3,4-thiadiazole compounds [13].
Altıntop et al. (2018) recently described the synthesis of 1,3,4-thiadiazole derivatives and assessed their anti-neoplastic activity against CML [14]. Schiff bases are compounds that contain an imine (–HC=N–) functional group. That produced by the condensation reaction of primary amines and carbonylcontaining compounds was first reported by Hugo Schiff [15– 17].
Sabbah et al. (2018) characterized the synthesis, and biological assessment of phenylimino-1,2- diphenylethanol derivative in colon carcinoma (HCT-116), and breast carcinoma (T47D) cell lines [18].
Shokrollahi et al. (2020) newly studied tetrahydro benzothiazolebased Schiff bases and assessed their cytotoxic activity against Hepatocellular carcinoma (HepG2) and human breast cancer (MCF-7) cell line [19].
Hazari et al. synthesized the complex of oxovanadium (IV), which represent a potent DNA cleavage activity and primary tumour reductions [20].
This study aimed to new synthesize 1,3,4-thiadiazole derivatives linked with different active groups with a potential affinity for binding DNA and could be used in the management of different cancer types.
Methods
Synthesis of 5-(1-(6-methoxynaphthalen-2-yl) ethyl)-1, 3, 4-thiadiazole-2-amine. Compound (I)
Compound (I) was synthesized based on the reaction between carboxylic acid (Naproxen) and thiosemicarbazide above phosphorous oxychloride (POCl3 ), As shown in equation (1) [21].
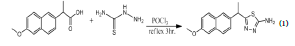
In a round-bottomed flask (100 ml), phosphorous oxychloride (3 ml) was added drop by drop to a cold mixture of (1 gm, 0.004 mole) carboxylic acid (Naproxen) powdered with thiosemicarbazide (0.37 gm, 0.004 mole) with string, the reflex was continued at 80°C for 3 hours, then cold to room temperature, poured to 250 ml of stirred iced-cold water and neutralized by 10% w/v NaOH solution. Then filtered the precipitate, was washed with water, and recrystallized from aqueous ethanol, the pale yellow powder was filtered out and leave it to dry at room temperature.
Synthesis of compound (a-h) Schiff bases derivatives
In a (50 ml) round-bottomed flask, an equimolar of compound (I) dissolved in 10 ml of absolute ethanol with an aromatic aldehyde, added 3 drops-5 drops of glacial acetic acid, reflex at 80°C for (3 hours-6 hours). The chemical reaction is summarized in equation (2). The reaction was monitored by using TLC. Ice-bath is used to cool the mixture until colored crystals form, the precipitate is filtered out and washed with cold ethanol, then recrystallization by ethanol and left to dry at room temperature [22]. The physicochemical properties of synthesized compounds were listed in table 1.
Tab. 1. Physicochemical properties of newly synthesized compounds
| Comp. | M.W | M.p. (ºC) | Appearances | Yield (%) |
|---|---|---|---|---|
| I | 285 | 160-163 | Yellow powder | 90 |
| IIa | 418 | 179-181 | Yellow crystals | 77 |
| IIb | 418 | 167-169 | Yellow crystals | 70 |
| IIc | 389 | 172-175 | White crystals | 65 |
| IId | 389 | 170-173 | White crystals | 73 |
| IIe | 373 | 162-165 | Yellow crystals | 80 |
| IIf | 403 | 189-192 | White powder | 76 |
| IIg | 403 | 186-188 | White crystals | 74 |
| IIh | 419 | 208-211 | White crystals | 86 |

R=a (2-NO2 ), b (4-NO2 ), c (3-OH), d (4-OH), e (H), f (3-OMe), g (2-Me), h (3-OMe, 4-OH)
Equation (2): The synthesis pathway of Schiff base derivatives.
Results
The newly synthesized compounds were prepared by conversion of Naproxen to 1,3,4- thiadiazole derivative linked to Schiff bases. The chemical structure of newly synthesized compounds was emphasized by using 1H-NMR, 13C-NMR, FT-IR, and C, H, N analysis. The physic-chemical properties of a compound are summarized in table 2.
Tab. 2. The spectroscopic analysis of newly synthesized compounds
| Comp. | Molecular Formula | The absorption band of FT-IR (cm-1) | The chemical shifts of 1H- NMR (ppm) | The chemical shifts of 13C-NMR (ppm) |
|---|---|---|---|---|
| I | C15H15N3OS | 3273-3433 w (NH2)3116 s (NH) | H1(3.8),H2(1.6),H3(4.5),H4(7.0),Arom.C-H (H5&H6) (7.2-7.8) | No signal of Schiff base |
| IIa | C22H18N4O3S | 1529 w (HC=N)1344 w (NO2) | H1(3.8),H2(1.6),H3(4.5)Arom.C-H(H5&H6) (7.2-7.9)N=CH(Imine)(10.50) | 190 (s, C=N )148 (s, C-NO2) |
| IIb | C22H18N4O3S | 1516 w (HC=N)1342 w (NO2) | H1(3.8),H2(1.6),H3(4.5) Arom. C-H (H5&H6) (7.2- 7.8)N=CH(Imine)(10.15) | 193 (s, C=N )151 (s, C-NO2) |
| IIc | C22H19N3O2S | 1557 w (HC=N)3267 w (OH) | H1(3.8),H2(1.6),H3(4.5)Arom. C-H(H5&H6)(7.2-7.8) N=CH(Imine)(9.90) | 193 (s, C=N )158 (s, C-OH) |
| IId | C22H19N3O2S | 1514 w (HC=N)3269 w (OH) | H1(3.8),H2(1.6),H3(4.5) Arom.C-H(H5&H6)(7.0- 7.75) N=CH(Imine)(10.0),O-H (6.4) | 190 (s, C=O )156 (s, C-OH) |
| IIe | C22H19N3OS | 1514 w (HC=N) | H1(3.8),H2(1.6),H3(4.5) Arom.C-H (H5&H6) (7.2-7.8) N=CH(Imine)(10.0), | 190 (s, C=N ) |
| IIf | C23H21N3O2S | 1557 w (HC=N)1052 s ( OCH3) | H1(3.8),H2(1.6),H3(4.5)Arom.C-H(H5&H6)(7.2-7.8), N=CH(9.90)O-CH3(3.6) | 193 (s, C=N )100 (s, OCH3) |
| IIg | C23H21N3O2S | 1514 w (HC=N)1029 s (OCH3) | H1(3.75),H2(1.6),H3(4.5) Arom.C-H (H5&H6) (7.0-7.75), O-CH3(3.6)N=CH(Imine)(10.30) | 193 (s, C=N )100 (s, OCH3) |
| IIh | C23H21N3O3S | 1514 w (HC=N)3255 w (OH),1028 s ( OCH3) | H1(3.8),H2(1.6),H3(4.5) Arom.C-H (H5&H6)(7.0-7.75), N=CH (9.75)O-CH3(3.6), O-H(6.5) | 191 (s, C=N )155 (s, C-OH)105 (s, OCH3) |
Biological activity
The half-maximal inhibitory concentration (IC50) of all synthesized compounds against both normal and cancer cells is summarized in table 3.
Tab. 3. IC50 values of newly synthesized compounds
| No | Compounds | IC50 ug.L-1 / Normal cell | IC50 ug.L-1 / cancer |
|---|---|---|---|
| 1 | IIb | 46.85 | 57.3 |
| 2 | IIf | 122.68 | 70.91 |
| 3 | IIa | 43.75 | 74.92 |
| 4 | IIg | 66.57 | 77.51 |
| 5 | I | 43.06 | 119.29 |
| 6 | IIe | 163.36 | 211.24 |
| 7 | IIc | 18.85 | 239.26 |
| 8 | IId | 70.54 | 568.78 |
| 9 | IIh | 84.93 | 1062.06 |
Discussion
The compound IIf show good cytotoxic activity with significant selectivity against cancer cell than normal cell. While other compounds IIa, IIb, and IIg lack selectivity toward cancer cells.
The compound IIb exhibit a potent cytotoxic activity compared with other compounds tested with comparable IC50 (57.30 ug/l), The compound IIa has lesser potency than compound IIb, both of them have a nitro group substituted at a different position on the phenyl ring. The nitro group at the para position reflects more potency than the ortho position of the same electron with drawing group.
Compound IIf shows a more potent cytotoxicity than compound IIg related to IC50 values respectively (70.91 ug/l, 77.51 ug/l). Both of them contain a methoxy group substituted on a different position on the phenyl ring. The meta position of IIf shows a most potent cytotoxic effect than the ortho position. The electron donating methoxy group on meta position exhibit good cytotoxicity with good selectivity toward cancer cells than normal cells as shown above IC50 for both normal and cancer cells.
The position of the substituted group (para, meta) plays an important role in the potency of the cytotoxic effect, while the substitution of the electron-donating group on meta position reflects the selectivity toward cancer cells over normal cells. The substitution of the electron-withdrawing group on the ortho position gives more potent cytotoxicity when compared to compound IIa and IIg containing nitro and methoxy group respectively at the same ortho position on the phenyl ring
Compound I (starting compound) show lower cytotoxicity compared with compound IIa, IIb, IIf, and IIg. The compounds IIe, IIc, IId. Show very lower cytotoxicity compared with both started and all above compounds containing phenyl ring only and hydroxyl group substituted respectively.
The compound IIh showed very low or no cytotoxic effect related to IC50 values for both normal and cancer cells. This compound contains both methoxy and hydroxyl groups substituted on the phenyl ring which lack their anticancer activity due to steric hindrance.
Conclusion
The newly synthesized compounds are successfully prepared depending on FT-IR, 1H-NMR, 13C-NMR, and C, H, N analysis data, and they revealed anti-neoplastic activity compared with standard Naproxen, They may be used in the management of various cancer diseases.
Funding
None.
Conflict of Interest
None.
References
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013; 501:328-37.
- Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013; 108:479-485.
- Aizawa K, Liu C, Tang S, Veeramachaneni S, Hu KQ, et al. Tobacco carcinogen induces both lung cancer and nonâ?alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int J Cancer. 2016; 139:1171-1181.
- Poon SL, McPherson JR, Tan P, Teh BT, Rozen SG. Mutation signatures of carcinogen exposure: genome-wide detection and new opportunities for cancer prevention. Genome Med. 2014; 6:1-4.
- Trafialek J, Kolanowski W. Dietary exposure to meat-related carcinogenic substances: is there a way to estimate the risk?. Int J Food Sci Nutr. 2014; 65:774-780.
- Cumberbatch MG, Cox A, Teare D, Catto JW. Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol. 2015; 1:1282-1290.
- Antwi SO, Eckert EC, Sabaque CV, Leof ER, Hawthorne KM, et al. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control. 2015; 26:1583-91.
- Seto M, Honma K, Nakagawa M. Diversity of genome profiles in malignant lymphoma. Cancer Sci. 2010; 101:573-578.
- Yamamoto K, Kawamoto S, Chijiki R, Watanabe M, Matsumoto S, et al. Biclonal diffuse large B-cell lymphoma commonly characterized by partial trisomy 18q involving MALT1 and BCL2. Intern. Med. 2023; 62:285-292.
- Roma-Rodrigues C, Fernandes AR, Baptista PV. Exploring RAB11A Pathway to Hinder Chronic Myeloid Leukemia-Induced Angiogenesis In Vivo. Pharmaceutics. 2023; 15:742.
- Wang J, Chen X, Ge X, Wang Z, Mu W. Molecular cloning, characterization and expression analysis of P53 from high latitude fish Phoxinus lagowskii and its response to hypoxia. Fish Physiol Biochem. 2022; 48:631-644.
- Dawood KM, Farghaly TA. Thiadiazole inhibitors: a patent review. Expert Opin Ther Pat. 2017; 27:477-505.
- El-Naggar M, Sallam HA, Shaban SS, Abdel-Wahab SS, E. Amr AE, et al. Design, synthesis, and molecular docking study of novel heterocycles incorporating 1, 3, 4-thiadiazole moiety as potential antimicrobial and anticancer agents. Molecules. 2019; 24:1066.
- Atmaram UA, Roopan SM. Biological activity of oxadiazole and thiadiazole derivatives. Appl Microbiol Biotechnol. 2022; 106:3489-505.
- Cimerman Z, MiljaniÄ? S, GaliÄ? N. Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat Chem Acta. 2000; 73:81-95.
- Raczuk E, Dmochowska B, Samaszko-Fiertek J, Madaj J. Different Schiff bases—structure, importance and classification. Molecules. 2022; 27:787.
- Awantu AF, Ayimele GA, Bankeu JJ, Nantia EA, Fokou PV, et al. Synthesis, Molecular Structure, Anti-Plasmodial, Antimicrobial and Anti-Oxidant Screening of (E)-1-(Phthalazin-1-yl)-1-[(Pyridin-2-yl) Ethylidene] Hydralazine and 1-[2-(1-(pyridine-3-yl) ethylidene) hydrazinyl] phthalazine. Int J Org Chem. 2021; 11:91-105.
- Sabbah DA, Al-Tarawneh F, Talib WH, Sweidan K, Bardaweel SK, et al. Benzoin schiff bases: Design, synthesis, and biological evaluation as potential antitumor agents. Med Chem. 2018; 14:695-708.
- Shokrollahi S, Amiri A, Fadaei-Tirani F, Schenk-Joß K. Promising anti-cancer potency of 4, 5, 6, 7-tetrahydrobenzo [d] thiazole-based Schiff-bases. J Mol Liq. 2020; 300:112262.
- Hazari PP, Pandey AK, Chaturvedi S, Tiwari AK, Chandna Set al. Synthesis of Oxovanadium (IV) Schiff base Complexes derived from Câ?substituted Diamines and Pyridoxalâ?5â?Phosphate as Antitumor Agents. Chem Biol Drug Des. 2012; 79:223-234.
- Mosa MN, Baiwn RS, Mohammed AK. Synthesis and Characterization of the Novel Compounds Containing Imidazole, Thiadiazole, Schiff Base, and Azetidinone Chromospheres as a New Antibacterial Agents. J Drug Deliv Technol. 2020; 10:602-607.
- Mousa MN. Synthesis, characterization and in vitro antioxidant activity of (1e, 4e)-1, 5-bis (4-hydroxyl-3-methoxyphenyl) penta-1, 4-dien-3-one. J Pharm Res. 2012 Feb; 5:913-914.



