Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 4
SIB-IMRT radiotherapy concomitant with cisplatin in locally advanced hypopharyngeal cancer: safety, feasibility
Lamiss Mohamed Sad*, Mohamed Barakat Khalil and Rasha Abd El-Ghany KhedrLamiss Mohamed Sad, Department of Clinical Oncology, Tanta University, Egypt, Email: lamismohamed@med.tanta.edu.eg
Received: 11-Apr-2021 Accepted: 23-Apr-2021 Published: 29-Apr-2021
Abstract
Introduction: Radiotherapy is the main line of treatment of head and neck cancer including hypopharyngeal cancer either adjuvant/neoadjuvant radiotherapy or neoadjuvant/adjuvant concurrent chemoradiotherapy. The aim of radiotherapy is to provide maximum safe dose to organ at risk (healthy normal tissues). Studies had shown that the need to increase dose to the tumour was associated with great hazard to healthy normal tissues with induction of acute and late toxicity. The acute toxicity will result in treatment breaks and decreased local control due to repopulation of the tumour cells especially in head and neck cancer. Aim of the study: It was to reduce the radiation dose received by critical normal organs to allowed tolerance. Materials and methods: Twenty-two hypopharyngeal cancer patients either receiving radiotherapy as definitive therapy with concurrent cisplatin in dose of 40 mgm/m2 (group A) or adjuvant radiotherapy concurrent with same dose of cisplatin (group B). The closest critical organs of interest were the eye lens, parotid gland, submandibular salivary gland, oral mucosa, and thyroid gland. The patients were treated with Simultaneous Integrated Boost (SIB) (2 Gy to 2.21 Gy) Intensity Modulated Radiation Therapy (IMRT) with total dose of 66 -70 Gy, 5 fractions per week. Results: Response after definitive treatment with concurrent simultaneous boost were 5 out of elven cases 45.5% achieved pathological complete response. Median overall survival is 33 months with range from 14 month-36 month for the whole group, with 2-year overall survival 63.3% and 81.8% in definitive and adjuvant group respectively which was statistically insignificant (p=0.457). Two-year local control 90.9% versus 63.6% in adjuvant versus definitive treatment which was statistical significance (p=0.034). Two-year larynx preservation survival was 63.3%. As regard the homogeneity index and confirmatory index, they were per guidelines to decrease the dose to organ at risk. No more than grade 2 xerostomia was reported in both treatment arms. Conclusion: these results showed high local control with simultaneous integrated boost IMRT with reduced dose to organ at risk and it was complementary to other studies regarding the new era of modern radiation therapy techniques.
Keywords
IMRT, OAR, hypopharynx, simultaneous integrated boost
Introduction
When human cells are subjected to ionizing radiation, it leads to deleterious effects on DNA. Among DNA damage, double strand DNA breaks are the most deleterious event for the side effects of radiotherapy [1-2].
The prognosis of hypopharyngeal cancer is poor and its treatment depends on stage. In early stage, radiotherapy and surgery give equivocal results while in advanced stage but operable disease, adjuvant radiotherapy or concurrent chemo radiotherapy increased tumour control but at the expense of side effects [3]. While inoperable cases, radical concurrent chemo radiotherapy is the main line of treatment but at the expenses of increased acute and late toxicities [4-5].
Intensity Modulated Radiation Therapy (IMRT) has been emerged as modern technique that increased local tumour control with reduced dose to organ at risk especially the salivary glands with decreased xerostomia risk [6-9].
Escalation of radiation dose had been used to improve the treatment outcome [10,11]. One the dose escalation technique is simultaneous integrated boost, which associated with increased local treatment control due to reduced repopulation [12-14].
So, the aim of our study was to evaluate simultaneous integrated boost IMRT/with without chemotherapy in locally advanced hypopharyngeal cancer as regard locoregional control, dosimetric study and toxicity in our institution.
Materials and Methods
Patients
This study was carried out at the oncology department; Tanta University in the period from January 2017 to December 2020, following written consent was taken from twenty-two locally advanced hypo pharyngeal cancer stage III and stage IVA patients according to TNM 8th edition [15-17]. The protocol was approved by the ethical committee.
Following panendosopy and biopsy of suspected lesion in the ENT department, Tanta university, patients were evaluated with thorough history taking, physical examination complete laboratory evaluation with special attention to complete blood picture, renal function, creatinine clearance and electrolytes, and radiological evaluation computed tomography of neck, MRI neck with contrast and PET- CT if indicated.
Treatment
For postoperative radiotherapy: Primary tumour and macroscopic suspicious lymph nodes diagnosed clinically and by imaging studies are delineated as the Gross Target Volume (GTV). High risk CTV1 was defined as tumour bed +0.5-1 cm and extracapsular nodal extension +0.5-1 cm, CTV2 was produced by GTV primary and nodal. Elective CTV3 is produced by elective nodal and adjacent tumour bed [18, 19]. To account for patient and treatment setup errors margin of 5 mm was added to each CTV to obtain planned target volume.
For definitive radiotherapy: CTV high disease GTV primary+5mm, GTV nodal +10mm; CTV high risk: tumour high risk areas and borderline lymph node and CTV low risk area adjacent to tumour and elective lymph node areas.
Patients are classified into two groups:
Group A: Concurrent chemo radiotherapy as primary treatment it was given in SIB IMRT dose of 7095 Gy/33 fxs, 2.15cGy/fx to PTV high disease (CTV high disease +5mm), 62.7Gy/33 fxs, 1.9Gy/fx for PTV high risk (CTV high risk+5mm) and 56.7Gy/33fx 1.7Gu/fx to PTV low risk (CTV low risk+5mm).
Group B: in postoperative group adjuvant concurrent chemo radiotherapy. the prescribed SIB IMRT was formed of 65Gy/30 fxs; 2.17/fx for PTV1 is formed by adding 5 mm to CTV1; for PTV3 formed by adding 5mm to CTV3 and the dose is 54Gy /30 fxs; 1.8 Gy/fx.
In both groups, patients received cisplatin in dose of 40 mgm/m2 weekly during radiotherapy with proper hydration. The energy used for treatment was 6MV photons using LINAC Linear accelerator, with multileaf collimators (UNIQUE) with dynamic multileaf collimator.
If patients lost weight more than 15%, nasogastric tube or per cutaneous gastrectomy were done.
Target (SIB and PTV) should be covered by 95% of prescribed dose. We evaluated the homogeneity index for both SIB and PTV as HI=(D1%-D99%) /D50%. Also, we record the conformity index for both SIB and PTV as CI=V95%2/(TV ⋅ PIV).
Dose volume histogram was used to evaluate organ at risk (parotid glands, spinal cord, brain stem, oral cavity) which are routinely contoured.
Follow up
Treatment follow up was done weekly during radiotherapy and month after end of treatment. In the first 2 years was done every month and every 6 months thereafter. Radiation therapy oncology group/European organization for treatment cancers defined acute toxicity as toxicity that occurred during radiotherapy or within 90 days after [20].
Statistical analysis
We used descriptive statistical using median, frequency. Locoregional control, overall survival and larynx preservation free survival was analysed using Kaplan-miere IBM statistics 21 and this was considered primary endpoints [21]. p value less than 0.05 was considered statistically significant. While the secondary endpoints were toxicity and adverse effects according to RTOG toxicity criteria [22].
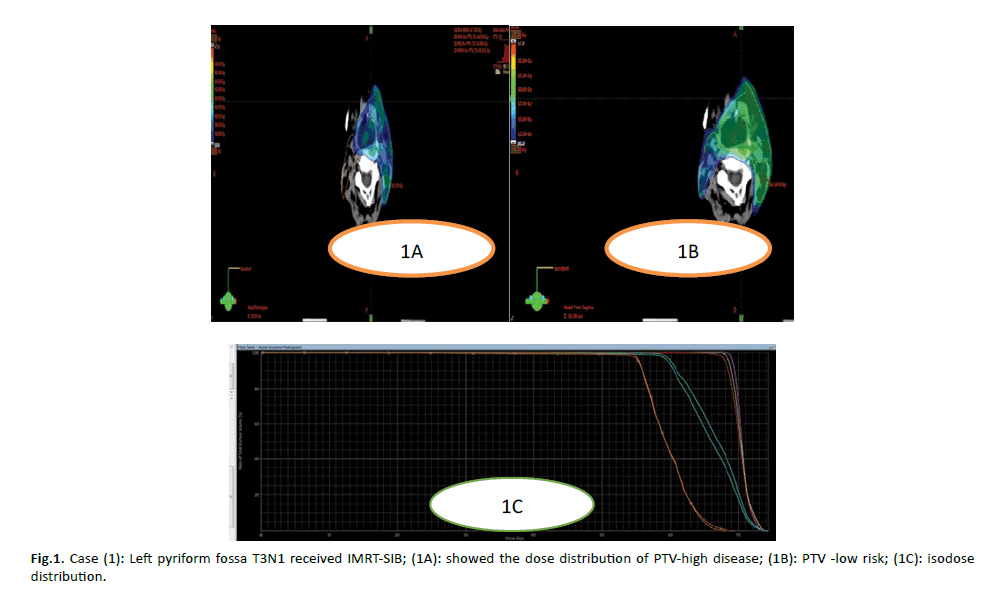
Figure 1: Case (1): Left pyriform fossa T3N1 received IMRT-SIB; (1A): showed the dose distribution of PTV-high disease; (1B): PTV -low risk; (1C): isodose distribution.
Results
Patients
Demographic data of 22 patients with locally advanced hypopharyngeal carcinoma stage III and IV A and divided in two groups: group A formed of elven cases received definitive concurrent chemo radiotherapy IMRT with simultaneous integrated boost while group B consisted of elven cases which received adjuvant chemo radiotherapy IMRT with SIB , and were summarized in table 1. No statistical difference as regard sex performance status, site either pyriform or postcorcoid squamous cell carcinoma in both treatment groups. But nine (81.8%) cases in definitive group (group A) were stage IVA while ten (90.9%) cases in adjuvant group (group B) were stage III which was statistically significant (0.01).
Tab. 1. Patients’ Demographic Information
| Parameter | Group A Definitive group | Group B Adjuvant group | Total | p value |
|---|---|---|---|---|
| Age | ||||
| Range ± SD | 40-70 +12.15 | 42-80 + 13.39 | 40-80 + 12.54 | |
| Median | 50 | 49 | 49 | |
| Sex | ||||
| Male | 10 (90.9%) | 9 (81.8%) | 19 (86.4%) | 0.543 |
| Female | 1 (9.1%) | 2 (18.2%) | 3 (13.6%) | |
| Cigarette smoking | ||||
| Cigarette smoking | 10 (90.9%) | 9 (81.8%) | 19 (86.4%) | 0.543 |
| Non-smoker | 1 (9.1%) | 2 (18.2%) | 3 (13.6%) | |
| Performance status | ||||
| 0-1 | 9 (81.8%) | 9 (81.8%) | 18 (81.8%) | 1 |
| 2 | 2 (18.2%) | 2 (18.2%) | 4 (18.2%) | |
| Stage | ||||
| III | 2 (18.2%) | 10 (90.9%) | 12 (54.5%) | 0.001 |
| IVA | 9 (81.8%) | 1 (9.1%) | 10 (45.5%) | |
| Site | ||||
| Pyriform | 8 (72.7%) | 8 (72.7%) | 16 (72.7%) | 1 |
| Postcircoid | 3 (37.3%) | 3 (37.3%) | 6 (37.3%) | |
Response after definitive treatment with concurrent simultaneous integrated boost were 5 out of elven cases 45.5% achieved pathological complete response Table 2.
Tab. 2. Response after definitive treatment with concurrent chemoradiotherapy arm (SIB-IMRT)
| Number | Percent | ||
|---|---|---|---|
| Response | CR | 5 | 45.5 |
| PR | 4 | 36.4 | |
| SD | 1 | 9.1 | |
| PD | 1 | 9.1 | |
| Total | 11 | 100 | |
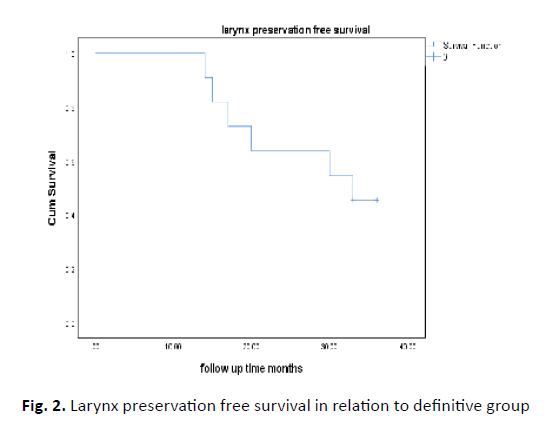
Six patients underwent salvage surgery two needed neck dissection and four needed both laryngectomy and neck dissection. Two patients needed tracheostomy during follow up period. Two-year larynx preservation survival was 63.3% Figure 2.
Figure 2: Larynx preservation free survival in relation to definitive group
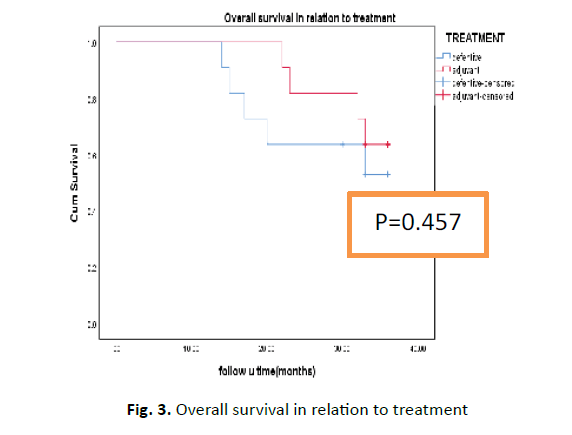
Median overall survival is 33 months with range from 14-36 month for the whole group, with 2-year overall survival 63.3% and 81.8% in definitive and adjuvant group respectively which was statistically insignificant (p=0.457) Figure 3. Univariate analysis showed no statistical significance except for pyriform fossa tumour which was associated with higher 2-year overall survival 87.5% versus 50% in PCC not statistically difference (P value was 0.21).
Figure 3: Overall survival in relation to treatment
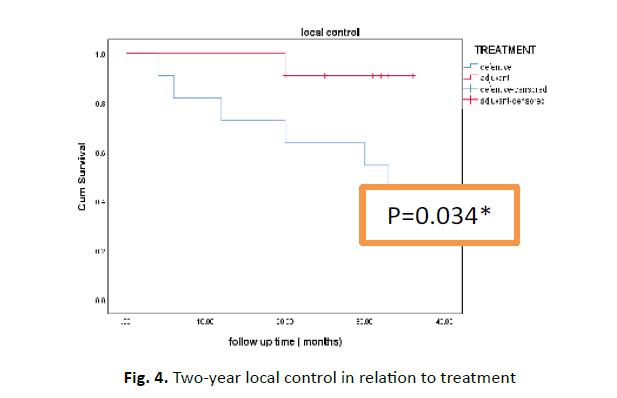
Two-year local control 90.9% versus 63.6% in adjuvant versus definitive treatment which was statistical significance figure (p=0.034) (Figure 4). Multivariate analysis showed no statistical significance of all prognostic factors (Table 3).
Figure 4: Two-year local control in relation to treatment
Tab. 3. Multivariate analysis of local control
| 95.0% CI for Exp (B) | ||||
|---|---|---|---|---|
| Parameter | Sig. | Exp(B) | Lower | Upper |
| Treatment definitive versus adjuvant | 0.115 | 0.101 | 0.006 | 1.745 |
| Sex male versus female | 0.337 | 7.356 | 0.125 | 432.817 |
| Ps0-1 versus 2 | 0.118 | 14.05 | 0.51 | 387.022 |
| Stage III VERSUS IVA | 0.922 | 1.135 | 0.092 | 14.053 |
| Site pyriform versus PCC | 0.919 | 0.911 | 0.152 | 5.477 |
Plan evaluation
We aimed at coverage of the planned target volume and simultaneous integrated boost with 95% of prescribed dose in both treatment arms. Table 4 showed the homogeneity index for PTV and SIB, it showed no statistical difference. It is defined (D1%-D99%) /D50%was calculated for SIB and PTV minus SIB. Confirmatory index was calculated by V95% 2/(treatment volume â?? planned integrated volume) and it was not statistically significant difference as regard both treatment groups. As regard organ at risk no statistically significant difference as regard mean dose to parotid D60% to oral cavity, spinal cord d 1c and brain stem D1cm as shown in Table 4.
Tab. 4. Plan evaluation
| Group A (Definitive group) | Group B (Adjuvant group) | p value | |
|---|---|---|---|
| PTV HI | 0.17 ± 0.045 | 0.18 ± 0.05 | 0.849 |
| PTV-SIB HI | 0.045 ± 0.036 | 0.038 ± 0.025 | 0.766 |
| PTV CI | 0.75 ± 0.373 | 0.82 ± 0.322 | 0.521 |
| SIB CI | 0.93 ± 0.21 | 0.93 ± 0.21 | 0.95 |
| Spinal cord D1cm | 36.07 ± 5.24 | 35.3 ±5.2 | 0.849 |
| Brain stem D 1cm | 43.6 ± 10.43 | 10.43 ± 43.10 | 0.783 |
| Parotid gland (left) | 23.29 ± 1.25 | 23.80 ±1.37 | 0.783 |
| Parotid gland (right) | 23.54 ± 1.61 | 23.29 ±1.25 | 0.893 |
| Oral cavity V60% | 31.45 ± 4.3 | 31 ± 4.39 | 0.958 |
PTV HI: Planned Target Volume Homogeneity Index, PTV-SIB: Planned Target Volume Simultaneous Integrated Boost, CI: Confirmatory Index
Toxicity
Mucositis grade 2 and grade 3 were observed in 12 cases (54.5%), 3 cases (13.6%) respectively. Ten cases (45.4%) developed xerostomia, of them grade 2 and grade 3 were observed in 2 cases (9.1%), and four cases (18.2) respectively. Grade 2 mucositis in the definitive group was higher than adjuvant group which was statistically significant (0.01) Table 5 No grade 3 late toxicity was observed. Only late grade 1 xerostomia was reported in 4 cases (18.2%) and grade 3 was reported in 2 cases (9.1%).
Tab. 5. Toxicity of both treatment groups
| Parameter | Group A | Group B | p value |
|---|---|---|---|
| Mucositis grade 2 | 9 (89.3%) | 3 (27.3%) | 0.01 |
| Mucositis grade 3 | 2 (18.2%) | 1 (9.1%) | 0.534 |
| Xerostomia grade 2 | 1 (9.1%) | 1 (9.1%) | 1 |
| Xerostomia grade 3 | 2 (18.2%) | 2 (18.2%) | 1 |
Discussion
The standard therapy for locally advanced head and neck cancer particularly hypopharynx is standard fractionation radiotherapy concurrent with cisplatin chemotherapy either single agent or combined with 5 fluorouracil or taxene chemotherapy [23-25]. This was associated with increased toxicity.
The introduction of new modalities of treatment such as IMRT and arc therapy lessen the toxicity associated with concurrent chemoradiotherapy especially salivary gland affection had to been introduced [3, 26-29].
In our study twenty-two patients with locally advanced hypopharyngeal cancer received concurrent cisplatin with intensity modulated radiotherapy with simultaneous. 11 patients in group A received treatment as definitive treatment, while elven patients received treatment as adjuvant treatment after surgery.
No statistical difference in patient demographic data between two groups of treatment like other [30-31]. Complete response in our series in patients received definitive IMRT-SIB with cisplatin was 45.5% and this was in accordance with huang et al. who reported complete response rate of 48% in which patients with locally advanced hypopharyngeal cancer received concurrent IMRT-SIB with cisplatin [30].
This in contrast to that reported in the study done by Franchin et al. in which patients with laryngeal cancer T3N0-1 or hypopharynx T2-4 N2-3 stage received induction chemotherapy followed by concurrent IMRT-SIB with cisplatin-5 fluorouracil, the complete response was 88.3% higher than reported by us due to addition of cancer larynx, induction chemotherapy and different concurrent chemoradiotherapy in that study [31]. Our complete response was also different from that reported by Liu et al being higher than that reported by us 82.5% because in that study patient with locally advanced hypopharyngeal cancer received IMRT-SIB concomitant with cisplatin and 5 fluorouracil [32].
In our study, two-year larynx preservation survival was 63.3% coincident with other authors [31-32], different from Huang et al., who reported that two laryngeal preservation rates of 50% that is lower than that reported by us and this may be due to 6% of cases in that trial was posterior hypopharyngeal wall but no cases in our trial [30].
In our study, two-year overall survival 63.3% and 81.8% in definitive and adjuvant group respectively which was statistically insignificant (p=0.457), this was similar to that reported by Harris et al. who reported that two-year overall survival was 66% and 77% in CRT and surgery plus radiotherapy and without chemotherapy non-significant [33]. And this was different from that reported by Huang et al. was reported higher two year overall survival in the CCRT group (55%) than that reported by adjuvant group (50%) which was statistically in significant( p=0.788) and this may be due to not all patients in the comparative study of adjuvant group received chemotherapy 50% only [30]. Univariate analysis of different prognostic factors with overall survival was statistically insignificant and this was similar to that reported by many other authors [30-33].
In our study, two-year local control 90.9% versus 63.6% in adjuvant versus definitive treatment which was statistical significance figure (p=0.034), and this was different from that reported by Huang et al higher 2-year locoregional group in the definitive group and statistically insignificant and may be due to only 50% of cases of adjuvant group received concomitant chemoradiotherapy [30].
Multivariate analysis of locoregional control in our study showed no statistically significant of age, sex, cigarette smokers, performance status, site of tumor, stage and treatment lines similar to other authors [30-33].
Our study showed that SIB-IMRT had produced accepted treatment plan for both lines of treatment either adjuvant or definitive line of treatment with acceptable dose to organ at risk as mentioned in results and this in line with that reported by many authors [10-14].
Conventional 3D conformal radiotherapy is associated with increased xerostomia; several studies had showed that IMRT has been used to reduce the dose to the salivary glands and resultant xerostomia [25, 33-34].
As our study compromised hypopharyngeal carcinoma, it was associated higher incidence of xerostomia due irradiation of submandibular lymph nodes with subsequent submandibular and minor salivary glands beside parotid glands. Grade 2 mucositis in the definitive group was higher than adjuvant group which was statistically significant (0.01) and no late grade 3 xerostomia was reported, only grade 1 xerostomia was reported in 4 cases (18.2%) and grade 3 was reported in 2 cases (9.1%). similar to that reported by other authors [35-36].
So, our study revealed that IMRT-SIB was associated improved 2 year overall survival, loco regional control which was higher in adjuvant group and improved larynx preservation free survival in the definitive group with reduced incidence of early late toxicity.
Study Limitations
The limitation of our study is the small sample size; more cases are needed to document the results and longer duration of follow up.
References
- Borrego-Soto G, Ortiz-López R, Rojas-Martínez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38:420-432.
- Todorovic V, Prevc A, Zakelj MN, Savarin M, Brozic A, et al. Mechanisms of different response to ionizing irradiation in isogenic head and neck cancer cell lines. Radiat Oncol. 2019;14:214.
- Vengaloor Thomas T, Nittala MR, Bhanat E, Albert AA, Vijayakumar S. Management of advanced-stage hypopharyngeal carcinoma: 25-year experience from a tertiary care medical center. Cureus. 2020 ;12:e6679.
- Iqbal MS, Chaw C, Kovarik J, Aslam S, Jackson A, et al. Primary concurrent chemoradiation in head and neck cancers with weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol. 2017;21:171-177.
- Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. 2017;22:1056-1066.
- Taylor A, Powell ME.Intensity-modulated radiotherapy--what is it? Cancer Imaging. 2004;4:68-73.
- Taylor A, Powell ME.Intensity-modulated radiotherapy--what is it? Cancer Imaging. 2004;4:68-73.
- Ghosh G, Gupta G, Malviya A, Saroj DJ Ghosh G, et al. Comparison three-dimensional conformal radiotherapy versus intensity modulated radiation therapy in local control of head and neck cancer. Cancer Res Ther. 2018;14:1412-1417.
- Mendenhall WM, Mendenhall CM, Mendenhall NP. Submandibular gland-sparing intensity-modulated radiotherapy. Am J Clin Oncol. 2014 ;37:514-516.
- Miah AB, Bhide SA, Guerrero-Urbano MT, Clark C, Bidmead AM, St Rose S, et al. Dose-escalated intensity-modulated radiotherapy is feasible and may improve locoregional control and laryngeal preservation in laryngo-hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2012;82:539-547.
- Gujral DM, Miah AB, Bodla S, Richards TM, Welsh L, et al. Final long-term results of a phase I/II study of dose-escalated intensity-modulated radiotherapy for locally advanced laryngo-hypopharyngeal cancers. Oral Oncol. 2014;50:1089-1097.
- Dobler B, Obermeier T, Hautmann MG, Khemissi A, Koelb O. Simultaneous integrated boost therapy of carcinoma of the hypopharynx/larynx with and without flattening filter - a treatment planning and dosimetry study. Radiat Oncol. 2017;12:114.
- Stromberger C, Ghadjar P, Marnitz S, Thieme AH, Jahn U, et al. Comparative treatment planning study on sequential vs. simultaneous integrated boost in head and neck cancer patients: differences in dose distributions and potential implications for clinical practice. Strahlenther Onkol. 2016;192:17-24.
- Dogan N, King S, Emami B, Mohideen N, Mirkovic N, et al. Assessment of different IMRT boost delivery methods on s on target coverage and normal-tissue sparing. Int J Radiat Oncol. 2003;57:1480-1491.
- Huang SH, O'Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18:40.
- Vallard A, Guy JB, Mengue Ndong S, Vial N, et al. Intensity-modulated radiotherapy or volumetric-modulated arc therapy in patients with head and neck cancer: Focus on salivary glands dosimetry. Head Neck. 2016;38:1028-1034.
- Brouwer CL, Steenbakkers RJ, Bourhis J, Budach W, Grau C, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015 ;117:83-90
- Grégoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol. 2006;79:15-20.
- Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93:219-222.
- Dragan T, Beauvois S, Moreau M, Paesmans M, Vandekerkhove C, et al. Clinical outcome and toxicity after simultaneous integrated boost IMRT in head and neck squamous cell cancer patients. Oral Oncol. 2019; 98:132-140.
- Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Intern J Radiat Oncol Biol Phys. 1995;31:1341-1346.
- Winquist E, Agbassi C, Meyers BM, Yoo J, Chan KW, et al. Systemic therapy in the curative treatment of head and neck squamous cell cancer: a systematic review. J Otolaryngol Head Neck Surg. 2017;46: 29.
- Dauzier E, Lacas B, Blanchard P, Le QT, Simon C, et al. Role of chemotherapy in 5000 patients with head and neck cancer treated by curative surgery: a subgroup analysis of the Meta-Analysis of Chemotherapy in Head and Neck Cancer. Oral Oncol. 2019; 95:106-114.
- Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58-S63.
- Johnston M, Clifford S, Bromley R, Back M, Oliver L, et al. Volumetric-modulated arc therapy in head and neck radiotherapy: a planning comparison using simultaneous integrated boost for nasopharynx and oropharynx carcinoma. Clin Oncol. 2011;23:503-511.
- Miyazaki M, Nishiyama K, Ueda Y, Ohira S, Tsujii K, et al. Preliminary analysis of the sequential simultaneous integrated boost technique for intensity-modulated radiotherapy for head and neck cancers. J Radiat Res. 2016;57:406-411.
- Teng F, Fan W, Luo Y, Ju Z, Gong H, et al. Reducing xerostomia by comprehensive protection of salivary glands in intensity-modulated radiation therapy with helical tomotherapy technique for head-and-neck cancer patients: a prospective observational study. Biomed Res Int. 2019;2019:2401743.
- Zhang YX, Peng HH, Zhang XX, Zhao JD, Wu WM, et al: A retrospective study on combined modality therapy with or without surgery for advanced hypopharyngeal squamous cell carcinoma: an analysis of 119 cases. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:352-358.
- Huang WY, Jen YM, Chen CM, Su YF, Lin CS, et al. Intensity modulated radiotherapy with concurrent chemotherapy for larynx preservation of advanced resectable hypopharyngeal cancer. Radiat Oncol. 2010;5:37.
- Franchin G, Vaccher E, Talamini R, Gobitti C, Minatel E, et al. Intensity-Modulated Radiotherapy with a Simultaneous Integrated Boost Combined with Chemotherapy in Stages III-IV Hypopharynx-Larynx Cancer: Treatment Compliance and Clinical Outcomes. J Radiotherp. 2014;2014:1-7.
- Liu WS, Hsin CH, Chou YH, Liu JT, Wu MF, et al. Long-term results of intensity-modulated radiotherapy concomitant with chemotherapy for hypopharyngeal carcinoma aimed at laryngeal preservation. BMC Cancer. 2010:10:102.
- Harris BN, Biron VL, Donald P, Farwell G, Luu QC, et al. Primary surgery vs chemoradiation treatment of advanced-stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141:636-640.
- Ghosh G, Tallari R, Malviya A. Toxicity profile of IMRT Vs. 3D-CRT in head and neck cancer: a retrospective Study. J Clin Diagn Res. 2016;10:XC01-XC03.
- DE Felice F, Pranno N, Papi P, Brugnoletti O, Tombolini V, et al. Xerostomia and clinical outcomes in definitive Intensity Modulated Radiotherapy (IMRT) versus Three-Dimensional Conformal Radiotherapy (3d-CRT) for head and neck squamous cell carcinoma: a meta-analysis. In Vivo. 2020;34:623-629.
- Fondevilla Soler A, López-Guerra JL, García Fernández A, Samaniego Conde MA, Belmonte González MJ, et al. Outcome and toxicity of intensity-modulated radiotherapy with simultaneous integrated boost in patients with pharyngo-laryngeal cancer. Clin Transl Oncol. 2019;21:881-890.