Review Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 9
Prognostic value of serum ferritin in cancerous patients
Hadeel Riyadh Ibrahim1*, Ahmed Jamal Jasim Alqaisi1 and Tarik M. Abdul Majeed Al-Bermani22Respiratory Department, AL-Karama Teaching Hospital, Ministry of Health/Environment, Baghdad, Iraq
Hadeel Riyadh Ibrahim, Hereditary Blood Diseases Center, AL-Karama Teaching Hospital, Ministry of Health/Environment, Baghdad, Iraq, Email: Masterr78@ymail.com
Received: 08-Sep-2021 Accepted: 30-Sep-2021 Published: 11-Oct-2021
Abstract
Cancers are the second cause of mortality worldwide. Ferritin is a known iron protein found in high concentrations in cancerous patients. The study aimed to estimate ferritin levels in cancerous patients, determine the correlation between serum ferritin and chemotherapy (CHX). A cross-sectional hospitalbased study was carried out in AlKarama Teaching Hospital. Assessments of the studied samples will be conducted as a baseline setting and in a subsequent manner. A total of 50 newly diagnosed cancer patients before undergoing CHX regimens (13 male and 37 female) were included. The kit Ferritin (FTL) Human ELISA Kit was used. In this study, 74% females and 36% males were studied. The mean age of the sample of the study was 50.85 ± 12.59 years. The breast cancer females about 40% of patients. About 51.3% of females, their ferritin concentration were within a normal level, while 28.2% of females have a high level. After all cycles of CHX, 75.6% of females have normal concentrations. These results have significant differences among pre and post CHX (ANOVA=1.992, p=0.049). Among males, the results have no significant among pre and post CHX (ANOVA=0.162, p=0.057). Among gender, at setting status, there was a high significant difference between high concentrations of ferritin and females cancers when compared with males cancer (p=0.039). The mean of ferritin concentration was dropped gradually with subsequent cycles of CHX (185.25 ± 47.283 μg/L)>(75.64 ± 49.699 μg/L)>(72.89 ± 38.42 μg/L)>(65.31 ± 29.187 μg/L), respectively, with a high statistical association (p<0.0001). The high level of ferritin is associated with advanced or untreated malignancies. CHX caused dropping in serum ferritin in subsequent cycles rather than a single cycle. A high level of ferritin is more recorded in female malignancies in comparison with a male.
Keywords
ferritin, cancer, chemotherapy, ferritin human ELISA kitt
Introduction
Cancers are a large topic of complex diseases that involve abnormal cell growth patterns [1]. This disease resulted from an interaction of genetic and internal factors with environmental factors, or cancer-causing agents [2]. It is the leading cause of death worldwide, accounting for 9.6 million deaths in 2018. Lung, breast, stomach, and colon cancer cause the most cancer deaths every year [1]. GLOBOCAN reported 18.1 million new cancer cases diagnosed in 2018 [3]. In Iraq, the newly registered cancer cases were more than 30 thousand that estimated by the latest Iraqi Cancer Registry report [4]. Chemotherapy (CHX) is a cancer treatment that uses one or more anti-cancer drugs or agents as part of a treatment regimen [5].
Ferritin is a known iron storage protein in intracellular compartments and it's level increase with age [6]. Iron overload, inflammation, liver disease, and malignancy are conditions that lead to elevated serum ferritin [7-11]. Pancreatic cancer, colorectal cancer, lung cancer, T-cell lymphoma, and hepatocellular carcinoma are associate with high serum ferritin [7-12].
Here, the level of serum ferritin estimating in the cancerous patients to determine the correlation between the serum ferritin in subsequent cycles of CHX, besides, establish the importance of ferritin as a prognostic agent in cancer management is of value.
Methods
Study design and setting
A cross-sectional hospital-based study was carried out on newly diagnosed patients with cancer in Al-Karama Teaching Hospital from 2nd February to 4th July 2020. Assessments of the studied samples will be conducted as a baseline before receiving CHX, designated as FF0, while the period after administration of the third cycle of CHX will be termed as FF1, FF3, and FF6.
Inclusion criteria
• All patients aged ≥ 18 years
• Patients who will in the first cycle of CHX
• Comfortable
Exclusion criteria
• Anticancer treatment that did not include CHX
• Patients in the second cycle of chemotherapy
• Unstable
Participants
A total 50 newly diagnosed cancer patients before undergoing CHX regimens (13 male and 37 female). Follow up will be recorded after the third cycle of CHX.
Samples collections
We collected blood into a serum separator tube. After clot formation, we centrifuged samples at 3,000 xg for 10 minutes and remove serum. Then diluted samples 1:10 into 1X Diluent M and assay.
The kit Ferritin (FTL) human ELISA kit
A Ferritin specific antibody has been pre-coated onto 96-well plates and blocked. Standards or test samples are added to the wells and subsequently, a Ferritin specific biotinylated detection antibody is added and then followed by washing with wash buffer. Streptavidin- Peroxidase Complex is added and unbound conjugates are washed away with wash buffer. TMB is then used to visualize Streptavidin- Peroxidase enzymatic reaction. TMB is catalysed by Streptavidin-Peroxidase to produce a blue colour product that changes into yellow after adding an acidic stop solution. The density of yellow colouration is directly proportional to the amount of Ferritin captured in the plate [13].
Procedure
Prepared all reagents, working standards and samples. Then, equilibrated reagents to room temperature before use. Then, removed excess microplate strips from the plate frame and returned them immediately to the foil pouch with a desiccant inside. Then, resealed the pouch securely to minimize exposure to water vapour and stored it in a vacuum desiccator. Then, added 50 μL of Ferritin Standard or sample per well. After that, tapped the plate to thoroughly coat the wells. Then, break any bubbles that may have formed. Then, covered wells with sealing tape and incubated for two hours. Then, started the timer after the last sample addition. After that, washed five times with 200 μL of 1X Wash Buffer manually. Inverted the plate each time and decant the contents; tapped it 4-5 times on an absorbent paper towel to completely remove the liquid. Then, added 50 μL of 1X Biotinylated Ferritin Antibody to each well. Tapped plate to thoroughly coat the wells. Break any bubbles that may have formed. Covered wells with sealing tape and incubate for one hour. Washed micro-plate as described above. Added 50 μL of 1X SP Conjugate to each well. Then, tapped the plate to thoroughly coat the wells. Break any bubbles that may have formed. Covered wells with sealing tape and incubate for 30 minutes. Turned on the microplate reader and set up the program in advance. Added 50 μL of Chromogen Substrate per well. Gently tapped plate to thoroughly coat the wells. Break any bubbles that may have formed. Incubated for 15 minutes or till the optimal blue colour density develop. Added 50 μL of Stop Solution to each well. The colour will change from blue to yellow. Gently tapped plate to ensure thorough mixing. Break any bubbles that may have formed. Then, read the absorbance on a microplate reader at a wavelength of 450 nm immediately [13].
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of Al-Karama Teaching Hospital. Informed written consent was obtained from all patients prior to their enrolment in this study.
Statistical Methods
Data of patients were entered and analysed using the statistical package for social sciences (SPSS, Chicago, US) version 25. Descriptive statistics are presented as mean, SD, frequencies and proportions. Statistical tests were applied according to the type of variables; ANOVA test for grouped samples was used to compare means of a continuous variable. Person’s correlation (2-sided) test used to compare grouped samples. A level of significance of ≤ 0.05 was considered as a significant difference or correlation.
Results
We enrolled 50 patients, 74% females and 36% males. The mean age of sample of the study was 50.85±12.59 years. The distributed age groups as followed: 30% for (41-50), 24% for (51-60), 22% for (61-70), 12% for (31-40), 6% for (>70), and 4% for (20-30). Most of patients were from Baghdad as 68%. About 76% of patients were jobless. Patients had past medical comorbid conditions were 50%. Regarding Body Surface Area (BSA), recorded 52% patients have BSA below 1.7 m2, while 26% of them were above 1.7 m2. The breast cancer females about 40% of patients. Other types presented as follow: 16% leukaemia’s 36% lung; 8% lymphomas. Regarding protocols, the AC+Taxen protocol was commonly used as 40%, followed by 36% Carboplatin+Taxen. (Table 1).
| Variables | n (%) | |
|---|---|---|
| Gender | Male | 13 |
| Female | 37 | |
| Age (years) | 20-30 | 2 |
| 50.85 ± 12.59 | 31-40 | 6 |
| 41-50 | 15 | |
| 51-60 | 12 | |
| 61-70 | 11 | |
| >70 | 3 | |
| Address | Baghdad | 34 |
| Diyala | 6 | |
| Wasit | 2 | |
| Babel | 4 | |
| Mosul | 4 | |
| Occupation | Employer | 12 |
| Non- employer | 38 | |
| Comorbidity | Present | 25 |
| Not | 25 | |
| Cancer types | Breast | 20 |
| Lymphoma | 4 | |
| Leukaemia | 8 | |
| Lung | 18 | |
| Chemotherapy* | 5FU+Cisplatin | 5 |
| AC+Taxen | 20 | |
| Carboplatin+Taxen | 18 | |
| ABVD | 4 | |
| MTX | 8 | |
| BSA (m2) | <1.7 | 26 |
| 1.7±0.88 | 1.7 | 11 |
| >1.7 | 13 | |
| Taxen: Paclitaxel or Docetaxel; AC: Adriamycin and Cyclophosphamide; MTX: Methotreaxate | ||
Tab. 1. Shows the themes, number of specific learning outcomes and contact hours
In setting status, the comparison between ferritin concentrations among CHX cycles done by ANOVA, whereas comparison between ferritin concentrations among gender done by Pearson’s correlation. 51.3% of females, their ferritin concentration were within the normal level. But 28.2% females have high concentration. After 1st cycle of CHX, 70% of female became within normal, while 6% still have high concentration of S. ferritin. When 3rd cycle performed, women within normal were 76%, whereas those with high level were 2%. At 6th cycle, 75.6% of female have normal concentration. These results have significant differences among pre and post CHX (ANOVA=1.992, p=0.049). Among males, the percent was normal in 17.9%, whereas it was high in 2.6% in the setting phase. The subsequent results as followed: FF1 (225 vs. 0), FF3 (22% vs. 0), and FF6 (22.2% vs. 0). These results have no significant among pre and post CHX (ANOVA=0.162, P=0.057).
Among gender, at setting status, there was a high significant differences between high concentrations of ferritin and females cancers when compared with males cancer (P= 0.039). After subsequent cycles of CHX, there was no association (P=0.054, 0.078, 0.1) (Table 2).
| Ferritin | FF0 (n=39) | FF1 (n=50) | FF3 (n=50) | FF6 (n=45) | ANOVA | p-value | |
|---|---|---|---|---|---|---|---|
| n (%) | |||||||
| Female | Low (<10 μg/L) | 0 | 1 (2) | 0 | 0 | 1.992 | 0.049 |
| Normal (10 μg/L-150 μg/L) | 20 (51.3) | 35 (70) | 38 (76) | 34 (75.6) | |||
| High (>150 μg/L) | 11 (28.2) | 3 (6) | 1 (2) | 1 (2.2) | |||
| Male | Low (<29 μg/L) | 0 | 0 | 0 | 0 | 0.162 | 0.057 |
| Normal (29 μg/L-248 μg/L) | 7 (17.9) | 11 (22) | 11 (22) | 10 (22.2) | |||
| High (>248 μg/L) | 1 (2.6) | 0 | 0 | 0 | |||
| p-value | 0.039 | 0.054 | 0.078 | 0.1 | |||
Tab. 2. Ferritin concentration in subsequent chemotherapy cycles
The mean of ferritin concentration was dropped gradually with subsequent cycles of CHX (185.25 ± 47.283 μg/L), (75.64 ± 49.699 μg/L), (72.89 ± 38.42 μg/L), (65.31 ± 29.187 μg/L), respectively. This was a statistically association (p=0.000) (Figure 1).
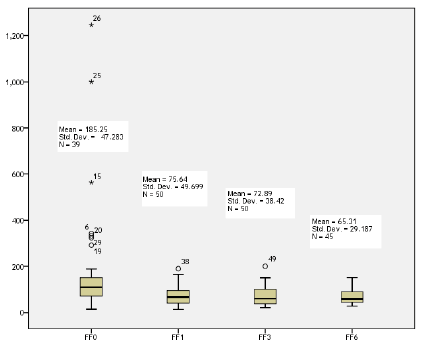
Figure 1: Box plot of ferritin concentrations in subsequent cycles of CHX
Discussion
Several studies reported increase serum ferritin levels in different types of cancer [7-12]. The high level of ferritin the poorer survival status in malignancy. These may be caused by an inflammatory process and regulatory effect on angiogenesis due to the secretion of ferritin by a tumour associated macrophage [14,15].
In this study, the level of ferritin in most patients was recorded within normal and some patients have high concentrations of ferritin before received CHX. These findings correlated with different previous studies [7 -12].
In previous clinical studies, the researchers found that the ferritin level correlated with the response rate to CHX in advanced NSCLC and breast cancer, and an elevated ferritin level was an independent prognostic factor for a poor survival outcome or prognostic biomarker [16-18].
This study showed that the mean concentration of serum ferritin was gradually decreasing in subsequent cycles of CHX, which could be explained by regressing of cancer activity and well response to the CHX. Khanna, et al. concluded that the concentration of ferritin was significantly higher in the advanced stage of cancer [19].
Conclusion
The high level of ferritin is associated with advanced or untreated malignancies. CHX caused dropping in serum ferritin in subsequent cycles rather than a single cycle. A high level of ferritin is more recorded in female malignancies than males. Good response rate according to ferritin level after completion of CHX regimens.
Conflict of Interest
None.
References
- WHO. Global Health Observatory. Geneva: World Health Organization. 2018.
- Burke KM, Mohn-Brown EE, LeMone PT. Medical Surgical Nursing Care. 3rd edition. Pearson. 2011.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Iraqi Cancer Registry. Ministry Of Health, Iraqi Cancer Board, Baghdad. 2018.
- Corrie PG, Pippa G. Cytotoxic chemotherapy: clinical aspects. Medicine. 2008;36: 24-28.
- Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98-104.
- Koyama S, Fujisawa S, Watanabe R, Itabashi M, Ishibashi D, et al. Serum ferritin level is a prognostic marker in patients with peripheral T-cell lymphoma. Int J Lab Hematol. 2017;39:112-117.
- Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, et al. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29:1905-1910.
- Ji M, Li XD, Shi HB, Ning ZH, Zhao WQ, et al. Clinical significance of serum ferritin in elderly patients with primary lung carcinoma. Tumour Biol. 2014;35:10195-1019.
- Kalousova M, Krechler T, Jachymova M, Kuběna A, Zak A,et al. Ferritin as an independent mortality predictor in patients with pancreas cancer. Results of a pilot study. Tumour Biol. 2012;33:1695-1700.
- Kowdley KV, Belt P, Wilson LA, Yeh M, Sanyal J, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77-85.
- Lee S, Song A, Eo W. Serum ferritin as a prognostic biomarker for survival in relapsed or refractory metastatic colorectal Cancer. J Cancer. 2016;7:957-964.
- Human NGAL(Neutrophil Gelatinase Associated Lipocalin) ELISA Kit. 2020.
- Alkhateeb AA, Han B, Connor JR. Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor associated macrophages. Breast Cancer Res Treat. 2013;137:733-744.
- Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013; 13:342-355.
- Lee S, Jeon H, Shim B. Prognostic Value of Ferritin-to-Hemoglobin Ratio in Patients with Advanced Non-Small-Cell Lung Cancer. J Cancer. 2019;10:1717-1725.
- Lee S, Eo W, Jeon H, Park S, Chae J. Prognostic Significance of Host-related Biomarkers for Survival in Patients with Advanced Non-Small Cell Lung Cancer. J Cancer. 2017;8:2974-2983.
- Jezequel P, Campion L, Spyratos F, Campone M, Andre J, et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in node-negative breast cancer tumors: A multi-centric 2004 national PHRC study. Int J Cancer. 2012; 131:426-437.
- Khanna V, Karjodkar F, Robbins S, Behl M, Arya s, et al. Estimation of serum ferritin level in potentially malignant disorders, oral squamous cell carcinoma, and treated cases of oral squamous cell carcinoma. J Can Res Ther. 2017;13:550-555.



