Case Report - Onkologia i Radioterapia ( 2024) Volume 19, Issue 2
Primary hepatic PEComa: A case report
Kelly Carter*Kelly Carter, Department of Surgery Nursing, Virginia Commonwealth University, Virginia, USA, Email: Carterkelly0102@gmail.com
Received: 26-Sep-2023, Manuscript No. OAR-24-114889; , Pre QC No. OAR-24-114889 (PQ); Editor assigned: 28-Sep-2023, Pre QC No. OAR-24-114889 (PQ); Reviewed: 12-Sep-2023, QC No. OAR-24-114889; Revised: 09-Oct-2024, Manuscript No. OAR-24-114889 (R); Published: 16-Oct-2024
Abstract
Mesenchymal neoplasms of the liver are very uncommon. As mesenchymal neoplasms, hepatic PEComas are part of the broader spectrum of rare liver tumors that arise from non-epithelial cell origins. Understanding the unique characteristics and clinical behavior of these tumors is crucial for early and accurate diagnosis, leading to appropriate treatment and improved patient outcomes. Herein, we present the case of a patient who underwent right hemihepatectomy with segment (IVA) preservation for a tumor identified as Fibrolamellar Hepatocellular Carcinoma (FL-HCC) by imaging investigations. The final histopathological examination revealed a Benign Epithelioid tumor (PEComa), with resection representing the standard of care. Clinical suspicion, the use of multiple imaging modalities, and a multidisciplinary approach are essential to avoid misdiagnosing unusual tumours such as hepatic PEComa. Additionally, long-term follow-up is crucial owing to the unpredictable natural history of PEComas.
Keywords
Mesenchymal neoplasms; Hepatic PEComas; Hemihepatectomy; Tumours
Introduction
Over the past few decades, advances in diagnostic techniques, particularly immunohistochemistry and molecular pathology, have significantly improved our ability to identify and classify perivascular epithelioid cell tumors (PEComas) and rare mesenchymal neoplasms that differentiate along a Perivascular Epithelioid Cell line (PEC) [1]. Originally considered a subset of smooth muscle tumors, the term "angiomyolipoma" was used to describe a subtype of PEComa that contains variable proportions of adipocytes, smooth muscle cells, and thick-walled tortuous blood vessels. Awareness of PEComas among clinicians and pathologists has increased, leading to an increased number of diagnosed cases worldwide. However, despite this progress, PEComas continue to pose significant diagnostic difficulties owing to their varied histological appearance and lack of specific genetic or molecular markers. While some PEComas exhibit indolent behavior and benign characteristics, others may demonstrate aggressive behavior with the potential for local recurrence or distant metastasis [2]. This heterogeneity necessitates a multidisciplinary approach to accurately stratify patients based on tumor characteristics and optimise treatment strategies.
Furthermore, the expanding spectrum of anatomical locations where PEComas can arise adds to the complexity of their management. Besides classical sites, such as the kidney and uterus, PEComas have been reported in various organs, including the liver, lungs, gastrointestinal tract, and soft tissues [3]. Each location may present unique challenges that require tailored therapeutic approaches.
Case Presentation
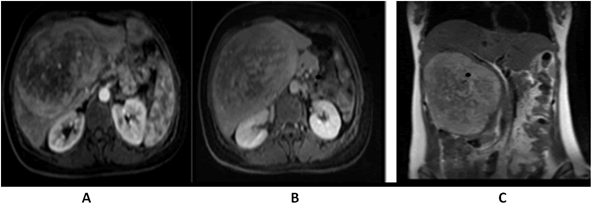
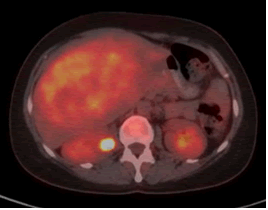
A 41-years-old woman was referred to our clinic complaining of fever, good performance (Eastern co-operative oncology group 1), no weight loss, no comorbidities, and no surgical history. On examination, there was a firm-to-hard, non-tender liver mass extending 13 cm below the right costal margin with rounded borders. All laboratory investigations, including liver function tests, viral markers, and tumor markers, were normal (alpha-fetoprotein< 1.3 ng/mL). Abdominal multidetector computed tomography revealed that the liver size was normal. Liver contours were smooth, and parenchymal density was normal. The hepatic veins, portal vein, and main branches were normal. The intrahepatic bile ducts were normal. A mass lesion, measuring approximately 15 × 11 cm, is observed near the level of liver segments V-VI-IVB, almost filling the space (HCC-fibrolamellar HCC?) No lymph nodes of abnormal size were observed in the abdomen. No significant free fluid is detected in the abdomen. The chest computed tomography findings were normal, the abdominal Magnetic Resonance Imaging (MRI) (Figure 1) showed at the level of segments IVB-V-VI, there is a heterogeneously hyperintense lesion measuring 16.5 × 14.8 ×10.2 cm on T2-weighted images, The lesion shows peripheral enhancement predominantly in the early post-contrast phase, followed by washout from the periphery in the delayed phase, with slightly increased enhancement in the centre, suggesting a possibility of Fibrolamellar Hepatocellular Carcinoma (FH-HCC). The main portal vein and right portal vein branches were compressed by the identified lesion. A Positron Emission Tomography (PET) scan was performed, which revealed a hypermetabolic mass lesion is detected in the liver, with no other lesions identified on the PET scan as the primary site (Figure 2), and the primary diagnosis was Fibrolamellar Hepatocellular Carcinoma (FL-HCC).
Fig. 1. Abdomen (MRI), A) (arterial phase) showing an enhancing lesion, B) (delayed phase) showing slight washout, C) (coronal T2 weighted) showed a heterogeneous mass in the right liver lobe.
Fig. 2. A Positron Emission Tomography (PET) scan revealed a hypermetabolic mass lesion in the right side of the liver.
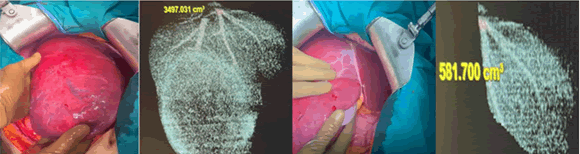
After a multidisciplinary discussion, the initial decision was to proceed with a liver transplant, given the characteristics of "Fibrolamellar Hepatocellular Carcinoma (FL-HCC) and large liver tumors with small left hepatic segments with a high risk for Post-Hepatectomy Liver Failure” (PHLF). The patient subsequently underwent right hemihepatectomy with segment VI preservation (Figure 3).
Fig. 3. Intraoperative imaging of the large right-side tumor with small left liver lobe, with liver volumetry for identical side.
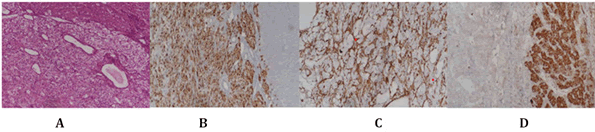
During gross examination of the specimen (Figure 4), cut sections revealed a well-circumscribed solid mass with a soft texture and gray-yellow color. The tumor and adjacent normal tissue boundaries were well-demarcated (expansive growth) and mainly consisted of two components: Epithelioid cells in the perivascular location and dilated thick-walled vessels. Epithelioid cells were characterized by clear to granular, lightly eosinophilic cytoplasm, and small, centrally located, normochromatic, round to oval nuclei. The nuclei showed slight variations and mostly demonstrated small nuclei. The cells had fairly distant cell borders and were arranged as nests and sheets. Mitotic figures and angiolymphatic invasion were not observed. Focal necrosis was observed, mostly with small nucleoli. In numerous sections, adipocytes were entirely absent. The adjacent liver tissue was microscopically normal immunohistochemically, and the tumor cells were positive for HMB-45, SMA, and Melan A and negative for arginase-1 and HepPar (Figure 5). Hepatic PEComa was diagnosed based on morphological characteristics and immunohistochemical results. The postoperative course was uneventful and the patient was discharged 10 days later.
Fig. 4. Cut sections revealed a well-circumscribed solid mass with a soft texture and gray-yellow color. Tumor and adjacent normal tissue boundary were well-demarcated (expansive growth).
Fig. 5. The neoplastic cells are epithelioid and characterized by clear to granular, lightly eosinophilic cytoplasm and small, centrally located, round to oval nuclei (5A). Most epithelioid tumor cells were markedly immunoreactive for HMB45 (5B), whereas SMA was focally positive (5C). The neoplastic HepPar cells were completely negative for IHC × 100 (5D).
Discussion
In recent years, there has been a noticeable rise in awareness and diagnosis of PEComa, an infrequent type of mesenchymal tumor. The number of reported cases of this rare condition has been steadily increasing. This upward trend highlights the growing recognition of PEComa as a distinct entity within the realm of rare tumors. A PEComa is a rare mesenchymal tumor composed of histologically and immunohistochemically distinct PECs. Bonetti et al., introduced PECs as a "unusual atypical cell type" with perivascular distribution and simultaneous melanocytic and myoid differentiation in 1992 [4]. The name PEComa was initially introduced by Zamboni [5-7]. PEComa can manifest in various anatomical sites, including the uterus, skin, and gastrointestinal tract. However, its occurrence in the liver is relatively rare [8,9] . According to the latest edition (5th edition) of the WHO 2020 classification epithelioid AML is a synonym for PEComa [10]. Due to the rarity of primary hepatic PEComa and its inconspicuous clinical presentation, its diagnosis poses a formidable challenge. From a clinical perspective, the reliable identification of hepatic lesions preoperatively heavily hinges on sophisticated imaging modalities. Notably, a considerable portion of cases presenting as hepatic PEComa are misdiagnosed as other hepatocellular malignancies, Particularly Hepatocellular Carcinoma (HCC), reflecting the intricate diagnostic landscape of this condition within the context of hepatic pathologies.
The primary challenge arises from the limited identification of specific imaging characteristics for this condition, which can be attributed to its rarity. In the context of liver ultrasound examinations, Computed Tomography (CT) scans, and Magnetic Resonance Imaging (MRI), it is not uncommon for tumors to be initially misidentified as Hepatocellular Carcinoma (HCC) [11,12]. Fibrolamellar Hepatocellular Carcinoma (FL-HCC) typically is single large tumor often displays distinctive features on Magnetic Resonance Imaging (MRI). hyperintensity on T2-weighted MRI sequences, primarily due to the presence of fibrous tissue within the tumor, resulting in increased water content and signal intensity. FL-HCC also typically exhibits robust arterial enhancement, appearing brighter during the arterial phase of contrast-enhanced MRI, owing to its rich blood supply. Moreover, FL-HCC tends to demonstrate delayed washout during the later phases of contrast enhanced MRI, distinguishing it from other liver lesions. Notably, a central fibrous scar is often observed within the tumor, appearing as a low-signal-intensity area on MRI-a hallmark feature of FLHCC. FL-HCC usually develops in non-cirrhotic liver tissue, setting it apart from other hepatocellular carcinomas associated with cirrhotic changes.
Due to the substantial histological diversity observed in hepatic PEComa, these tumors frequently deviate from displaying typical imaging characteristics, Ultrasonography commonly reveals a distinct round lesion that presents as hyperechoic in up to 90% of cases, often demonstrating marked vascularization. In addition, the tumor does not show MR-specific features; it is usually hyperintense in T2-weighted images and hypointense in T1-weighted images and may show restriction of water diffusion. A recently introduced technique, Contrast-Enhanced Ultrasonography (CEUS) utilizing Sonazoid, has emerged as a promising method for identifying PEComa noninvasively, thereby holding potential for facilitating its diagnosis [11] in a recently published study by Gao, Xuedong et al. [12], This review analyzed 11 cases of hepatic PEComa, focusing on the characteristics of imaging results, such as conventional ultrasound, CDFI, contrast-Enhanced Ultrasound (CEUS), and CECT. The results revealed hypodense lesions, hyperechoic patterns, abundant blood supply, clear margins, hyperechoic patterns, lateral shadows, large blood vessels, and enhancement patterns. These features may indicate expansive tumor growth and aid in the diagnosis of PEComa. Ultrasound imaging may provide additional details for clinical reference.
In our specific case, the patient presented with fever. Other reported symptoms include nausea, abdominal pain, indigestion, and loss of appetite. However, it's noteworthy that many patients either remain asymptomatic or manifest vague gastrointestinal symptoms. Furthermore, in certain instances, fever might even serve as the primary complaint [13].
Krawczyk Marek, et al., found that on reviewing 20 PEComas, the vast majority were women, in most patients in literatures, the tumor is accidentally detected during ultrasound testing for other reasons. None of these patients had a history of parenchymal liver disease. Laboratory test results were normal. Serum AFP, carcinoembryonic antigen, and Ca 19-9 concentrations were also within the normal limits as our patient.
Most PEComas exhibit benign characteristics; however, aggressive PEComas that demonstrate potentially invasive growth with malignant potential and metastatic risk have been reported. Malignant transformation is more likely with tumor size >5 cm, an infiltrative growth pattern, a high nuclear grade, mitotic activity >1 per 50 high-power fields, and evidence of necrosis [14].
Microscopically, the identification of epithelioid or spindleshaped cells containing adipocytes serves as a suggestive indicator of the disease. Further confirmation of the diagnosis involves an analysis of specific biomarkers, including HMB-45, Melan-A, and SMA. A novel marker, TFE3, is of recent interest due to its strong expression in 15% of PEComas, indicative of TFE3 gene rearrangements. However, our current knowledge of the molecular genetics underlying PEComas remains limited. Notably, two distinct molecular groups have been discerned: One characterized by mutations in the TCS1 and TSC2 genes, and the other linked to TFE3 fusions, designated as TFE3-translocation associated PEComas. It's worth highlighting that the TSC1 and TSC2 genes are involved in the negative regulation of mTOR activation. As a result, mTOR inhibitors have emerged as a potential palliative therapeutic strategy for PEComas, particularly in patients harbouring TSC mutations.
Further exploration into the oncogenic role of TP53 in PEComa is warranted, as the exact mechanistic interactions remain to be fully elucidated. Intriguingly, there have been reported cases of liver PEComa diagnosis occurring concurrently within families afflicted by Li-Fraumeni syndrome. This intriguing association raises the possibility of TP53 alterations contributing to the pathogenesis of PEComa, highlighting the necessity for in-depth molecular investigations to unravel the intricate underlying pathways driving the initiation and progression of this uncommon mesenchymal neoplasm.
Following the initial Multidisciplinary Discussion (MDT), the primary treatment option for the patient was liver transplantation. This decision was made based on the initial imaging findings, which raised suspicion of Fibrolamellar Hepatocellular Carcinoma (FL-HCC). Additionally, the patient's liver size was relatively small, indicating that extended right hemihepatectomy could potentially lead to a small future remnant liver size and pose challenges in preserving adequate liver function post-surgery. The possibility of FL-HCC and the concern about the small and inadequate Future Remnant Liver (FRL) size led the MDT to lean towards liver transplantation as a curative option.
However, it's noteworthy that when the patient was prepared for liver transplant and a biopsy was ordered to confirm the diagnosis. The patient was a nurse, expressed strong reluctance to undergo a liver biopsy. She cited concerns and potential risks associated with the procedure.
The case was re-evaluated considering additional clinical data and further investigation. Upon re-evaluation, it was noted that the patient had no underlying liver disease and had a normal liver background. Liver function test results, viral serology, and other laboratory results were within the normal range, indicating a healthy liver. This new information raised doubts about the initial diagnosis of FL-HCC and prompted the MDT to rediscuss the case. In the subsequent MDT meeting, we considered the patient's young age, previously healthy liver, and absence of underlying liver disease. Moreover, a thorough review of the imaging findings of the tumor was conducted. The decision to proceed with right hepatectomy while preserving segment IVa was based on feasibility and achievement of clear surgical margins. The decision to perform surgery was based on the results of the imaging studies. We assumed that the patients had indications for resection and that a biopsy would not change our decision since patient diagnosed as (FL-HCC). Based on the current few reported cases, surgical intervention continues to be the optimal and successful approach for treating primary liver PEComa. Both anatomical and nonanatomical resections have demonstrated the ability to achieve clear margins and favourable outcomes.
The patient underwent a right hemihepatectomy with segment (IVA) preservation. It is noteworthy that, although minimally invasive as laparoscopic resection is a viable option for liver resections, our surgical team has experience with open surgical techniques. Therefore, we chose the open surgical approach, liver function tests remained within normal limits, indicating that the preserved liver segments functioned well and effectively compensated for the resected portion. Laboratory investigations did not reveal any signs of liver dysfunction or other abnormalities. On the tenth day after surgery, the patient's condition significantly improved, and she was discharged from the hospital with a favourable prognosis.
The consideration of a watch-and-wait strategy has emerged in discussions regarding the management of benign hepatic PEComa diagnosed, guided by Contrast-Enhanced Ultrasonography (CEUS) modalities. Given the frequent misdiagnosis of PEComa as alternative lesions and the limited utilization of preoperative biopsies, However, it’s important to acknowledge that we have limited long-term data about treating hepatic PEComas, especially when considering the watch-and-wait strategy.
Systemic therapy is the treatment of choice in cases of disseminated malignant PEComas, considering the potential effectiveness of mTOR inhibitors. There are very few reports on the nonsurgical treatment of patients with malignant liver PEComas. In rare situations, transarterial chemoembolization and ablation have been used.These nonsurgical methods should be considered in cases in which surgery is contraindicated.
Since PEComa's prognosis can be unpredictable, we will closely follow our patient over the long term, following our institution's guidelines for primary liver tumors.
Conclusion
To avoid misdiagnosis of rare tumors like hepatic PEComa, a combination of clinical suspicion, tumor on normal liver, female sex, normal liver laboratory tests, advanced imaging techniques like contrast-enhanced ultrasound (CEUS) and Contrast-Enhanced CT (CECT), immunohistochemistry, liver biopsy, multidisciplinary collaboration, and long-term follow-up are needed. Using these comprehensive strategies can improve diagnostic accuracy and treatment planning.
References
- Algashaamy K, Montgomery EA, Garcia-Buitrago M. Liver mesenchymal neoplasms: Something old, something new. Pathol. 2022; 54:225-235.
[Crossref] [Google Scholar] [PubMed]
- Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. The Am J Surg Pathol. 2005; 29:1558-1575.
[Google Scholar] [PubMed]
- Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992; 16:307.
- Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, et al. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. Am J Roentgenol. 2014; 202:252-258.
[Crossref] [Google Scholar] [PubMed]
- Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, et al. Clear cell “sugar” tumor of the pancreas: A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996; 20:722-730.
[Crossref] [Google Scholar] [PubMed]
- Ameurtesse H, Chbani L, Bennani A, Toughrai I, Beggui N, et al. Primary perivascular epithelioid cell tumor of the liver: New case report and literature review. Diagn Pathol. 2014; 9:1-7.
[Crossref] [Google Scholar] [PubMed]
- Khaja F, Carilli A, Baidas S, Sriharan A, Norford S. PEComa: A perivascular epithelioid cell tumor in the liver-a case report and review of the literature. Case Rep Med. 2013; 2013:904126.
[Crossref] [ Google Scholar] [PubMed]
- Tan Y, Zhang H, Wang XC . Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres. Indian J Pathol Microbiol. 2014; 57:453-455.
[Crossref] [Google Scholar] [PubMed]
- Zhao LJ, Yang YJ, Wu H, Huang SM, Liu K. Perivascular epithelioid cell tumor of the liver: A case report and literature review. Eur Rev Med Pharmacol Sci. 2013; 17:1665-1668.
[Google Scholar] [PubMed]
- Krawczyk M, Ziarkiewicz-Wroblewska B, Wroblewski T, Podgorska J, Grzybowski J, et al. PEComa-A rare liver tumor. J Clin Med. 2021; 10:1756.
- Li C, Xu JY, Liu Y. Sonazoid-enhanced ultrasonography and pathologic characters of CD68 positive cell in primary hepatic perivascular epithelioid cell tumors: A case report and literature review. Open Med. 2021; 16:737-741.
[Crossref] [Google Scholar] [PubMed]
- Gao X, Tang H, Wang J, Yao Q, Wang H, et al. Specific imaging features indicate the clinical features of patients with hepatic perivascular epithelioid cell tumor by comparative analysis of CT and ultrasound imaging. Front. Oncol. 2022; 12:908189.
[Crossref] [Google Scholar] [PubMed]
- Song Z, Xu F, Dai C. Chills and fever as the first presentation of hepatic perivascular epithelioid cell tumor. Hepatobiliary Surg Nutr 2019; 8:436.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Wang Q, Zhou X, Zhou H, Jia W, et al. Retrospective analysis of hepatic Perivascular Epithelioid cell tumour (PEComa) in a single centre for clinical diagnosis and treatment clinical diagnosis and treatment of hepatic PEComa . Med. 2022; 101:e29506.
[Crossref] [Google Scholar] [PubMed]