Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 1
Neoadjuvant sequential short course radiotherapy and folfox versus neoadjuvant long course chemoradiation and evaluation of glascow scale sensitivity as a prognostic and predictive marker in locally advanced rectal cancer
Fatma Soliman El Sahi1*, Wael Mansour Saeed1, Lamiss Mohammed Abdelaziz1, Gamal Ibrahim Mousa2 and Samar Galal Younis12Department of Gastro-intestinal and Laparoscopic Surgery, Faculty of Medicine, Tanta University, Egypt
Fatma Soliman El Sahi, Department of Clinical Oncology & Nuclear medicine, Faculty of Medicine, Tanta University, Egypt, Tel: 01095582447, Email: atma.elsahy@med.tanta.edu.eg
Received: 07-Jan-2023, Manuscript No. OAR-23-86137; Accepted: 30-Jan-2023, Pre QC No. OAR-23-86137 (PQ); Editor assigned: 10-Jan-2023, Pre QC No. OAR-23-86137 (PQ); Reviewed: 24-Jan-2023, QC No. OAR-23-86137 (Q); Revised: 29-Jan-2023, Manuscript No. OAR-23-86137 (R); Published: 31-Jan-2023
Abstract
Background: The trial hypothesis was that short-course radiotherapy followed by near-total neoadjuvant chemotherapy would increase patient adherence to treatment compared to standard chemo-radiotherapy for locally advanced rectal cancer, without affecting oncological results.
Methods: In this open-label RCT, patients with cT3, cT4, or node-positive non-metastatic rectal cancer were randomly assigned to receive complete mesorectal excision after receiving either 5*5 Gy of radiation and FOLFOX or concomitant chemo-radiotherapy and capecitabine. Adjuvant chemotherapy cycles were administered to patients using the FOLFOX regimen. The primary endpoint was to evaluate the response rate after the completion of treatment. The secondary goal was to assess the failure rate (local or distant) and treatment toxicity.
Results: Of 100 patients, 50 were in arm 1 and 50 in arm 2. Patients’ characteristics differences between the two groups were not significant except for mutant BRAF and pre-treatment CEA levels p=0.043 and p<0.001. Tumour regression grade 1 was 18% in arm 1 and 2% in arm 2. The pathological response was statistically significant with BRAF mutation (p<0.001), pretreatment CEA level (p=0.006), treatment arms (p=0.031), and pre-treatment GPS (p<0.001). For Overall Survival (OS), the 3-year OS was 94% which was statistically insignificant between the two arms (p=0.564). Three years OS was higher in patients with GPS zero, normal pre-treatment CEA level, wild-type BRAF mutation, and TRG 1 and 2.
For disease-free survival, the 3-year DFS was 90% that was statistically insignificant between the two arms (p=0.627). Three-year DFS was higher in TRG1 and 2 100% for both, normal pre-treatment CEA level and pre-treatment GPS zero.
The chemotherapy adverse events were vomiting, anaemia, and neurotoxicity which were almost the same in both arms 1 and 2. As regard radiation therapy side effects, the main genitourinary toxicity in the form of dysuria and urinary tract infection46% in arm 1 and 66% in arm 2 (p=0.292), lower GIT toxicity proctitis occur in 40% in arm 1 and 30% of arm 2 (p=0.022) with no grade 3 or grade 4 toxicity in both arms.
Conclusion: Short course radiotherapy followed by chemotherapy (near total neoadjuvant therapy) then TME offers comparable results.
*Watch and wait strategy may be the appropriate option in patients developing a complete pathological response.
Keywords
short course radiotherapy, near total neoadjuvant, locally advanced cancer rectum, glascow prognostic scale
Introduction
Colorectal cancer is the third most frequently diagnosed cancer and the fourth leading cause of cancer death in the United States [1]. The American Cancer Society’s estimates for the number of colorectal cancer cases in the United States for 2017 are 95,520 new cases of colon cancer and 39,910 new cases of rectal cancer [2]. In Egypt, colorectal cancer showed low incidence and a high proportion of young-onset disease [3]. In patients with clinical T1 and T2 with N negative rectal cancer, the standard treatment is primary surgery without preoperative therapy [4]. In Locally Advanced Rectal Cancer (LARC) (clinical T3-4 with any N negative or N positive with any T), Involvement of the Meso Rectal Fascia (MRF) has a strong prognostic impact on survival and local recurrence rates. A conventional treatment plan for these individuals involves preoperative long-course Con-Current Chemo-Radiation (CCRT), Total Mesorectal Excision (TME), and adjuvant chemotherapy. Aimed at Tumour shrinkage and achieving R0 resection [5]. In patients with LARC, Short-Course Radiotherapy with Delayed Surgery (SCRTDS) is known to be a valuable therapeutic option. As compared to traditional Neoadjuvant Radio-Chemotherapy (NRC), SCRTDS leads to similar results in terms of the rate of R0 resection and satisfactory results in terms of down staging and pathological response so, can be considered in patients with locally advanced tumours, especially those who are unfit for chemo-radiation [6]. In respectable rectal cancer, randomized trials conducted in Poland and Australia compared preoperative short-course irradiation (5 Gy) and prompt surgery with preoperative long-course chemo-radiation and delayed surgery. Long-term results in either experiment did not differ [7]. Preoperative 5*5 Gy and immediate surgery were contrasted with 5*5 Gy and delayed surgery in a Stockholm III randomized research. A preliminary study revealed Tumour down staging in the group receiving delayed surgery [8]. These results and information from the literature led researchers to the hypothesis that long-course chemo-radiation would not be as effective as short-course chemo-radiation if surgery is postponed after 5*5 Gy and consolidation chemotherapy are administered between 5*5 Gy and surgery [7,8]. The benefit of the shortcourse schedule is a lower rate of early toxicity than with chemo radiation. In addition, short-course irradiation is less expensive and more convenient, especially in centres with long waiting lists [9]. Accurate prediction of the prognosis of rectal cancer will help in tailoring appropriate therapy [10]. Long-term survivors of rectal cancer especially with advanced stages remain low. A lot of prognostic factors affect survival in rectal cancer such as age, performance state, pathological stage, and grade [11]. The prognosis of the tumour didn’t depend upon these features alone but on host inflammatory response as well [12]. Inflammation and malignant illness are closely related to one another. ColoRectal Cancer (CRC) has been linked to elevated levels of C-Reactive Protein (CRP), a sign of systemic inflammation [12]. A simple assessment of the blood CRP and albumin levels yields the Glasgow Prognosis Score (GPS), an inflammationbased prognostic score, which has been demonstrated to be a helpful prognostic indicator in colorectal cancer as cancer-related inflammation can affect tumour cell motility, invasion, metastasis, cell survival, angiogenesis, and the ability of the immune system to respond. [13].
Methods
At a tertiary-care academic university hospital in Egypt, a prospective, interventional, open-label clinical study with a single institution was conducted. The research was conducted in conjunction with the Departments of Clinical Oncology and Nuclear Medicine and Gastrointestinal & Laparoscopic Surgery. The protocol (registration number 32139) was accepted by the institutional ethics board, and the trial was conducted in compliance with the international standards for good clinical practice. Patients needed to be fit enough for major surgery and chemotherapy, have a previously untreated primary rectal adenocarcinoma in the upper, middle, or lower rectum, and have a clinical UICC TNM stage of II (T3 or T4 node-negative) or III (T3 or T4 node-positive). Before enrolling in the experiment, every eligible patient provided their informed permission. Exclusion standards included: distant metastases, pulmonary, renal, or hepatic major organ failure, a history of cancer, prior pelvic irradiation, peripheral neuropathy, or a cerebral stroke.
Preoperative assessment and staging included a colonoscopy, pelvic MRI, CT abdominal, chest CT, and serum Carcino Embryonic Antigen (CEA), C-RP measurement, and serum albumin to assess Glascow prognostic Scale Physical examination and radiological imaging were used to define clinical staging at baseline. Consecutive patient enrolment took place, and MDT meetings were used to make treatment decisions.
This research was performed at the Department of Clinical Oncology & Nuclear medicine, Faculty of Medicine, Tanta University. Ethical Committee approval and written, informed consent were obtained from all participants.
Treatment
In a 1:1 ratio, patients were randomly assigned to either SRT or 4-cycle FOLFOX (arm 1) or long-course CRT (arm 2). At allocation, patients were told of their treatment strategy. In an MDT meeting, patients were discussed both at baseline and right before surgery. Before surgery, patients in arm 1 underwent preoperative radiation with a dosage of 25 Gy administered in five fractions of 5 Gy each (5*5 Gy) over five days. This was followed by four cycles of chemotherapy FOLFOX. In arm 2, the dose of radiotherapy was 50.4 Gy in 28 fractions at 1.80 Gy per fraction, delivered at five fractions in a week over 5 weeks-5.5 weeks. Details of the radiotherapy technique are available below. Patients in arm 1 underwent Total Mesorectal Excision (TME) at 9 weeks-10 weeks after completion of radiotherapy while 4 patients refuse to go for surgery, whereas those in arm 2 underwent TME at 6 weeks8 weeks after radiotherapy The Stockholm III and Polish trials provided the basis for the decision to delay surgery after SRT, f inding that patients did better in terms of the tumour down staging and PCR. In contrast, the addition of chemotherapy to SRT was intended to address micro metastatic disease early in the course of treatment. It is debatable whether surgery should be performed following CRT, however any time between 4 weeks to 12 weeks is still advised; in actuality, the higher end of the range yields a superior pCR9. To balance the resolution of radiation side effects, surgery was performed in arm 2 in 6 weeks to 8 weeks following radiotherapy (Figure 1). In arm 1, chemotherapy consisted of a 2-hour infusion of oxaliplatin (100 mg/m2) and a 2-hour infusion of leucovorin (CF) (400 mg/m2) on Day 1 and a 46-hour infusion (2.4 g/m2) repeated every 14 days. Unless restricted or limited by radiation toxicities, in which case it was begun once such toxicities had resolved, this was intended to be started about 7 days after radiotherapy had finished. When chemotherapy had to be delayed due to side effects, every effort was taken to keep the time between chemotherapy and surgery as close to the protocol as feasible. Based on the findings of two significant RCTs, chemotherapy was chosen as the post-SRT treatment in arm 1 [14]. During irradiation, patients in arm 2 received capecitabine twice a day at a dosage of 1650 mg/m2. The choice to forego oxaliplatin in this arm was made because of the potential for increased toxicities without an increase in clinical benefit [15]. Patients' toxicity and general treatment tolerance were evaluated weekly during radiation and again after treatment was complete. The full radiation courses for the SRT arm lasted one week, and patients had evaluations after the procedure. The National Cancer Institute of the USA's Common Terminology Criteria for Adverse Events, version 4.0, was used to classify acute toxicities. Preoperative MRIs were performed on all patients, along with regular assessments for metastatic disease to determine operability and forecast the condition of the Circumferential Resection Margin (CRM). Surgery was either an abdominoperineal resection or a sphincter-saving operation (low anterior resection/ultralow anterior resection). Microscopically free margins, including the CRM, were used to define R0 resection and were expressed as a percentage of patients receiving radiation in each arm. The capacity to undertake a sphinctersaving operation in a patient who would otherwise have needed an abdominoperineal resection with the sacrifice of the sphincter due to low tumour placement or levator involvement was referred to as sphincter preservation. Postoperative complications were categorized according to the Clavien-Dindo system and were classified as those that occurred within 30 days of surgery [16]. The resection specimen's quality was assessed following predetermined standards. The lack of tumour cells in the removed main Tumour and lymph nodes were what was known as a PCR (up T0 N0 ). To measure tumour regression in response to neoadjuvant treatment, the 5-grade Menders grade was utilized [17]. The FOLFOX regimen's adjuvant chemotherapy cycles for each patient were planned to be administered at the aforementioned dosages.
Radiotherapy technique
Patients preparations
Before treatment, proper staging and nutritional evaluation were done for all patients.
Patients simulation
1. Pre-simulation bowel preparation to ensure an empty rectum in the form of laxatives, glycerine suppository, or enema.
2. Simulation in the prone position with radiopaque marker on the anus.
3. CT simulation with 3 mm thickness
4. Full comfortable bladder filling
5. Daily kilo-voltage portal images were done in the short course arm and weekly in the long course arm.
Treatment volume
In short course arm:
*IMRT radiotherapy technique, including CTV (primary tumour, mesorectum, presacral lymph nodes, and lymph nodes along internal iliac vessels up to sacral promontory and lymph nodes at obturator nodes) (Figure 1).
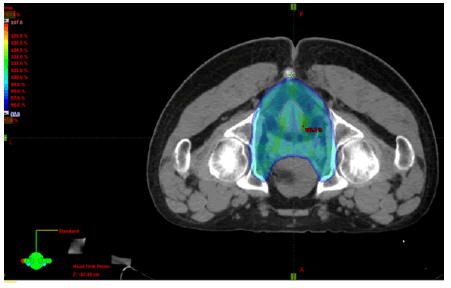
Figure 1: Dose distribution for a case of locally advanced rectal cancer received 500 cGY*5 fraction
In long course arm:
GTV primary is defined as all gross disease on physical examination and imaging (CT, MRI, PET-CT) and colonoscopy (Figure 2).
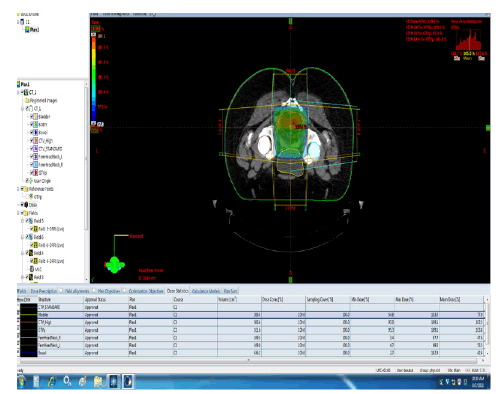
Figure 2: Dose distribution for a case of locally advanced rectal cancer received 5040 cGY*28 fraction. The main OAR considered for all patients include small bowel, large bowel, bladder, femoral heads, and external genitalia had been delineated and doses reach to them did not exceed their tolerance
GTV regional nodes are all visible perirectal and involved iliac nodes.
1. CTV A includes the rectum, and meso-rectum carrying primary Tumour with perirectal, presacral, and internal iliac lymph nodes. Should be covered for all patients.
2. CTV B includes the external iliac nodes (covered only in T4 disease).
3. CTV C includes the inguinal lymph nodes (only in cases that extend into the distal anal canal or lower third vagina).
4. Boost dose to 50.4 Gy include GTV primary and positive lymph nodes.
PTV is generally a 0.5-1 cm expansion of all the CTVs to account for potential setup errors and patient motion.
The objectives in long course plans were as follows:
1. Small bowel: V40Gy<100cc.
2. Bladder: Dmax<50 Gy,
3. Femoral heads: Dmax<50Gy.
The objectives in short course plans were as follows
1. Small bowels no more than 200 cc to receive more than 20 Gy.
2. Bladder no more than 35% to receive more than 22 Gy.
3. Femoral heads no more than 40% to receive more than 15 Gy.
Treatment planning
Treatment planning was done on the Eclipse planning system which has been configured for photons for IMRT using Varian Medical Systems as a linear accelerator.
3D-CRT and IMRT were planned in two to three sequential phases (summed to get the composite plan) to a total Tumour dose of 25 Gy in arm 1 and 50.4 Gy-54 Gy in arm 2 using a planning process.
Beam Energy
IMRT using 6 MV to 15 MV photons symmetrical planning target volume margins of 1 cm-2 cm
Dose and Fractionation
Arm 1:
500 cGy in 5 daily fractions
Arm 2:
50.4 Gy-54 Gy in 28-30 fractions of 1.8 Gy including 3D-conformal boost dose to the High Risk-Planning Target Volume (HR-PTV)
Plan evaluation
Dose-Volume Histograms (DVHs) of the GTV and CTV and the critical normal structures, including the small bowels, bladder, and femoral neck were obtained (Figures 3 and 4).
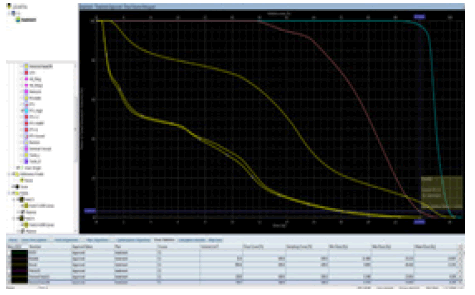
Figure 3: Dose volume histogram for a case of locally advanced rectal cancer received 500 cGY*5 fraction
Plan quality was analysed from (DVH) data. The treatment goal for each patient was to deliver 95% of the prescribed dose to ≥ 95% of the PTVs with a maximum dose limited to 107% of the prescribed dose).
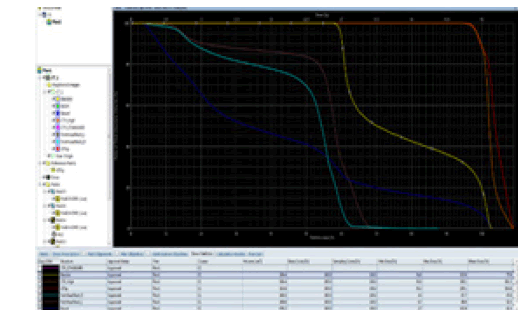
Figure 4: Dose volume histogram for a case of locally advanced rectal cancer received 5040 cGY*28 fraction
The aim of the treatment plan design and optimization process is to deliver the prescribed dose to the target while limiting the dose to normal structures.
The homogeneity index as well as the confirmatory index was calculated for every plan.
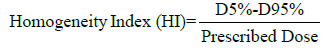
Where:
D5% and D95% are the received dose by 5% and 95% of the target volume.
HI=0 is the most acceptable value. The closer to zero the better dose homogeneity.
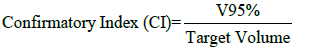
Where:
V95% is the volume of PTV covered by at least 95% of the prescribed dose.
CI=1 is the most acceptable value. If CI>1, it means irradiating volume is greater than the target volume and includes healthy tissue.
Treatment interruptions
Treatment interruption was avoided as much as possible through close monitoring of the patients and proper medications to overcome any troubles.
Follow-up
For the first two years, follow-up was scheduled every three months; thereafter, it was done every six months. At each followup appointment, a physical examination and a CEA estimate were performed as assessments. After the first and second years, colonoscopy, abdominal and pelvic CT, and chest CT or radiography were all advised. Late complications were rated using the Radiation Therapy Oncology Group scale developed by the European Organization for Research and Treatment of Cancer [18].
Statistical Analysis
Compliance with therapy corresponds with better results and was thus chosen as the primary endpoint in light of the oncological parity between SRT and CRT. The capacity to finish prescribed treatment, such as the previously mentioned adjuvant chemotherapy to a scheduled dose of twelve cycles of folfox, surgery, and neoadjuvant radio-chemotherapy, was referred to as treatment compliance. On Saturday through Wednesday, our unit administers radiotherapy (5 fractions per week). The SPSS statistical computer tool version was used to arrange, tabulate, and statistically analyse the acquired data. The Chi-Square test was used to compare the features of the patients; significance was determined by two-tailed P values of 0.05. P values between 0.05 and 0.10 were considered to be on the edge of statistical significance. The Cox proportional hazards model was utilized for multivariate analysis, and the Kaplan-Meier technique was employed to determine survival plots and cumulative survival probability. From the time of diagnosis through the time of disease recurrence and/or distant metastases, Disease-Free Survival (DFS) was determined. From the date of diagnosis until the date of death or the final follow-up, Overall Survival (OS) (actual survival) was computed [19].
Results
Between January 2018 and May 2020, 100 patients were assigned randomly to arms 1 and 2. Baseline characteristics are shown in Table 1. The median serum CEA levels at baseline were 2.8 (3-152) ng/ml in arm 1and 2.5 (3-321) ng/ml in arm 2. After neoadjuvant therapy, they fell to 2 (1-8) and 1.5 (2-7) ng/ml respectively. Pretreatment GPS 0 was 92% in Arm 1 and 82% in Arm 2.
Tab. 1. Correlation of baseline patients’ and tumor characteristics between arm 1 and arm 2
| nTNT arm | Total | Chi square | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | X² | P-value | ||
| Age | Median (range) | 45 (27-68) | 43 (30-70) | 0.938 | |||||
| Gender | Male | 29 | 58.00% | 33 | 66.00% | 62 | 62.00% | 0.679 | 0.41 |
| Female | 21 | 42.00% | 17 | 34.0%9 | 38 | 38.00% | |||
| PS | 0 | 40 | 80% | 35 | 70% | 75 | 75% | 0.143 | 0.705 |
| 1 | 10 | 20% | 15 | 30% | 25 | 25% | |||
| Clinical Stage | T3 N0 | 15 | 30.00% | 14 | 28.00% | 29 | 29% | 0.474 | 0.976 |
| T3 N1 | 14 | 28.00% | 14 | 28.00% | 28 | 28% | |||
| T3 N2 | 4 | 8.00% | 6 | 12.00% | 10 | 10% | |||
| T4 N0 | 13 | 26.00% | 12 | 24.00% | 25 | 25% | |||
| T4 N1 | 4 | 8.00% | 4 | 8.00% | 8 | 8% | |||
| T4 N2 | 0 | 0.00% | 0 | 0.00% | 0 | 0% | |||
| Grade | Grade 2 | 31 | 62.00% | 29 | 58.00% | 60 | 60% | 0.167 | 0.683 |
| Grade 3 | 19 | 38.00% | 21 | 42.00% | 40 | 40% | |||
| Distance from anal verge | Low | 13 | 26.00% | 14 | 28.00% | 27 | 27% | 0.067 | 0.967 |
| Mid | 20 | 40.00% | 20 | 40.00% | 40 | 40% | |||
| High | 17 | 34.00% | 16 | 32.00% | 33 | 33% | |||
| Kras/Nras | Mutant | 13 | 26.00% | 14 | 28.00% | 27 | 27% | 0.051 | 0.822 |
| Wild | 37 | 74.00% | 36 | 72.00% | 73 | 73% | |||
| BRAF | Mutant | 2 | 4.00% | 3 | 6.00% | 5 | 5% | 0.211 | 0.646 |
| Wild | 48 | 96.00% | 47 | 94.00% | 95 | 95% | |||
| Pre-treatment CEA (ng/mL) | Median (range) | 2.8 (0-223) | 2.5 (0-850) | 0.49 | |||||
| GPS pre | 0 | 46 | 92% | 41 | 82% | 87 | 87% | 3.065 | 0.216 |
| 1 | 2 | 4% | 7 | 14% | 9 | 9% | |||
| 2 | 2 | 4% | 2 | 4% | 4 | 4% | |||
Radiotherapy, Chemotherapy, And Toxicities
5*5 Gy of radiation were planned for each subject in arm 1. Patients in arm 2 were slated to receive 50.4 Gy of radiation over 28 daily portions. The most frequent side effect of neoadjuvant treatment was haematological, with anaemia occurring in 96% of patients in arm 1 and 98% of patients in arm B (P=0.749) (Table 2). The prevalence of diarrhoea was higher in arm 2 (any grade: 26% in arm 1 vs 40% in arm 2; P=0.015), necessitating dose adjustment for radiation in 3 patients and chemotherapy in 5 patients. Every patient in arm 1 finished their radiation at the prescribed dose. Nevertheless, three patients in arm 1 needed dosage adjustments for chemotherapy (4% in arm 1 vs 7% in arm 2; P=0.719). Overall, 2% in arm 1 and 4% in arm 2 experienced acute gastrointestinal toxicities ranging in severity from grade III to IV (P 1.000; HR 0.69, 0.12 to 3.98). Following the completion of radiation, chemotherapy was begun in arm 1 on average 7 (6 -7) days later. Nine patients (13%) in arm 1 had their chemotherapy delayed by 3 (2-4) days, with seven patients experiencing acute toxicities and two experiencing organizational issues. In the second arm, one patient exhibited grade I haematuria. In comparison, 32 patients (45%) in arm B suffered radiation treatment delays, with 16 (23%), exceeding the approved treatment schedule by more than one week (P=0.001; HR=0.19, 0.09 to 0.43), while radiotherapy treatment delays were less prevalent in arm 1. Treatment times for neoadjuvant therapy were also lower in arm A than arm B, including the interval to surgery (P<0.001).
Tab. 2. Adverse events in treatment arms 1 and arm 2
| Adverse events | Arm 1 | Arm 2 | p-value all grades | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1-4 | Grade3/4 | G1-4 | Grade 3/4 | |||||||
| N | % | N | % | N | % | N | % | |||
| Chemotherapy adverse events | Neutropenia | 30 | 60 | 0 | 0 | 35 | 70 | 0 | 0.558 | |
| Anemia | 48 | 96 | 2 | 4 | 49 | 98 | 1 | 0.749 | ||
| Peripheral neuropathy | 43 | 86 | 0 | 0 | 48 | 96 | 0 | 0 | 0.197 | |
| Vomiting | 44 | 88 | 0 | 0 | 48 | 96 | 0 | 0 | 0.311 | |
| Radiotherapy adverse events | Genitourinary toxicity | 23 | 46 | 0 | 0 | 33 | 66 | 0 | 0 | 0.292 |
| Lowergittoxicity diarrhea | 13 | 26 | 0 | 0 | 20 | 40 | 0 | 0 | 0.015 | |
| Proctatitis | 20 | 40 | 0 | 0 | 15 | 30 | 0 | 0 | 0.022 | |
Outcomes After Surgery
Tumours were not resected in four patients (8%) in arm 1 as they refused surgery after achieving a complete pathological response in neoadjuvant treatment. The majority of patients received a laparoscopic or open sphincter-saving treatment (low or ultralow anterior resection). Between the study groups, there were no changes in the rates of R0 resection, sphincter preservation or pCR, your status, or ypT stage (Table 3). The SRT arm had greater down staging of the main Tumour (ypT category). Overall, 75% of patients in both groups had Tumour down staging (P=0.920; HR=1.01, 95% CI=0.83 to 1.22). There were no differences in postoperative complications between the two treatment arms (P is ¼ of 0.838) tumour regression grade 1(patient who achieved CR) was 18% in arm 1 and 2% in arm 2. The rest of the patients develop grades of response in arms 1 and 2 with 2% of patients in arm 2 having no response which was statistically significant (p=0.031).
Tab. 3. Correlation between tumor regression grade according to treatment arms
| TRG | Arm 1 | Arm 2 | Chi-square | |||
|---|---|---|---|---|---|---|
| N | % | N | % | X² | p-value | |
| CR | 9 | 18.00% | 1 | 2.00% | 10.647 | 0.031* |
| Presence of rare residual cancer cell | 21 | 42.00% | 18 | 36.00% | ||
| Increase number of residual cancer cell but fibrosis still prominent | 16 | 32.00% | 20 | 40.00% | ||
| Residual cancer outgrowing fibrosis | 4 | 8.00% | 10 | 20.00% | ||
| Absence of regressive changes | 0 | 0.00% | 1 | 2.00% | ||
There were no significant differences for the pathological response as regards age, sex, performance status, clinical stage, grade, distance from the anal verge, and K-ras/N-ras mutation, while it was statistically significant with BRAF mutation (p<0.001), pretreatment CEA level(p=0.006), treatment arms (p=0.031) and pre-treatment GPS (p<0.001).
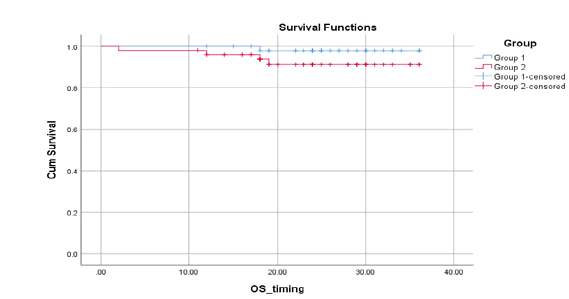
For overall survival (OS), the 3-year overall survival was 94% with a median follow-up period of 20 months ranging from 12 to 38 months. Three years OS was 96% in arm 1 versus 92% in arm 2 which was statistically insignificant (p=0.564) (Figure 5). Three years OS was higher in patients with GPS zero 100%which was highly statistically significant (p<0.001).
Figure 5: Correlation between 3-year OS with treatment arms 1 and arms 2
Three-year OS was significantly better with normal pre-treatment CEA level, 100%, wild type BRAF mutation 97.3%and TRG 1 and 2 100% for both (p<0.001 for each), but no significant differences were found as regard age, gender, performance, clinical stage, grade, distance from the anal verge, K-ras/N-ras mutation and treatment arms.
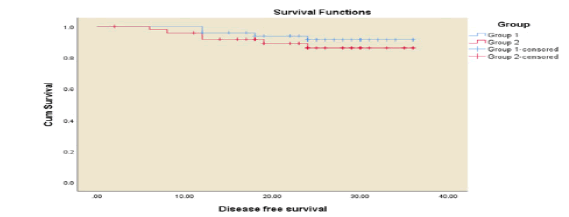
For disease-free survival, the 3-year DFS was 90% with a median follow-up of 20 months ranging from 12 to 38 months. Three-year DFS was 92% in arm 1 versus 88% in arm 2 which was statistically insignificant 9p=0.627 (Figure 6). Three-year DFS was higher in TRG1 and 2 100% for both, with normal pre-treatment CEA level 100% and pre-treatment GPS zero 100% (p<0.001 for each), but no significant differences were found as regards gender, performance, clinical stage, grade, distance from the anal verge, K-ras/N-ras and BRAF mutation and treatment arms.
Figure 6: Correlation between 3 years’ disease free survival and treatment arms
Treatment Compliance
The SRT arm had superior overall compliance with the prescribed course of action. After surgery, all patients in arms 1 and 2 completed planned adjuvant chemotherapy for a total of 12 cycles. T he most common chemotherapy adverse events were vomiting, anaemia, and neurotoxicity which were almost the same in both arms 1 and 2. Grade ¾ anaemia was more in arm 1 (p=0.749). Peripheral neuropathy was more in arm 2 (96%) (p=0.197) vomiting was more in arm 2 (96%) vs (88%) in arm 1 (p=0.311) with no recorded grade ¾ vomiting or peripheral neuropathy in both arms. As regard radiation therapy side effects, the main radiation-associated toxicity was genitourinary toxicity in the form of dysuria and urinary tract infection46% in arm 1 and 66% in arm 2 (p=0.292).
According to lower GIT toxicity proctitis occurs in w 40% of arm 1 and 30% of arm 2 (p=0.022) with no grade 3 or 4 toxicity in both arm.
Prognostic Factors
There were no significant differences in pathological response concerning age, sex, performance status, clinical stage, grade, distance from the anal verge, and K-ras/N-ras mutation, while it was statistically significant with BRAF mutation (p<0.001), pretreatment CEA level(p=0.006), treatment arms (p=0.031) and pre-treatment GPS (p<0.001).
Discussion
This prospective, interventional, open-label clinical trial showed improved treatment compliance for SRT, with no difference in rates of R0 resection, sphincter preservation, pCR, tumour down staging, or postoperative complications, or incidence of acute of neoadjuvant therapy between the two groups. Median age for the entire study population was 42 from 27 years-70 years, this indicates that many people have rectal cancer with an early beginning. Due to underlying genetic changes, young-onset rectal cancer is a distinct subtype with atypical tumour biology. The goal of treating these patients should be to cure them with vigorous neoadjuvant therapy, lessen the necessity for a permanent stoma, reduce long-term side effects, and improve their quality of life [20]. The majority of the participants in the current research had cT4 tumours, clinical nodal positive, and threatened margins, which are indicators of advanced rectal cancer. Although initial tumour down staging was much better in the SRT arm of the experiment, acceptable down staging, sphincter preservation, and R0 resection were nevertheless seen in both trial arms. The data from the current experiment are compared with data sets from other pertinent studies using SRT in Table 3, and the outcomes are comparable [14, 21].
Rectal cancer multimodal treatment has changed throughout time. From a quick procedure within a week to a delayed surgery, SRT delivery has changed. Although the old method sterilized the meso-rectum and made surgical margins easier, waiting until after surgery causes tumour down staging due to potential immune-mediated processes [22]. Recent studies found no differences in oncological outcomes between SRT and CRT in two sizable randomized trials. The most recent recommendations from the European Society for Medical Oncology (ESMO) [23]. Consequently, permit SRT in all prognosis categories of rectal cancer, with the "advanced" group requiring the addition of chemotherapy to SRT. The incidence of neoadjuvant therapy's acute toxicity varies between the two arms. at level of lower gastrointestinal toxicity each arm had different toxicity profile but none of them had grade ¾ toxicities None of the toxicity differences between the 2 arms were statistically significant except for lower GIT radiation related toxicity as proctitis was higher in arm 1 and diarrhea was higher in arm 2. Similar to Markovina who reported that acute gastrointestinal and all non-hematological toxicities were similar in both cohorts as were postoperative and late toxicities [24].
Also Chakrabarti, et al, did not found any significant difference between toxicities of both arms as anaemia was seen in 28% of patients in arm A and 32% of patients in arm B, making it the most frequent side effect of neoadjuvant treatment in either arm. Diarrhea was more prevalent in arm B (any grade) than arm A (two patients in arm A during chemotherapy and one in arm B during CRT). Arm B required dose change for chemotherapy in five patients and radiation in three patients, whereas arm A required dose modification for both. The prescribed dose of radiation was completed by all patients in arm A. Three patients in arm A, however (4% in arm A versus 7% in arm B), required dosage adjustment for chemotherapy. Overall, 2% in arm A and 4% in arm B experienced acute grade III-grade IV gastrointestinal toxicities [25].
Also Ngan, et al observed no significant difference in severe late toxicity at 3 years and, in particular, no reports of severe neuropathy [14]. In the present study, pathological complete response occurred in 9 (18%) of patients in arm 1, which was more than pCR in Polish trial (16%) and Chakrabarti et al. (12%) rate of PCR in Rapid trial which may be due to administration of different regimen of chemotherapy and more cycles chemotherapy preoperative 6 cycles Xelox in Rapido trial [25-27]. In arm 2 the rate of PCR was 1 (2%) and rate of all achieved response TRG 1, 2, 3 and 4 was occurred in (78%) in comparison with Markovina et al., 2017 who reported that pathological complete response occurred in 28% in nTNT arm and 16% in NCRT arm increasing rate of pCR in arm 2 may be due to approximately one third of patients in arm 2 received multi-agent concurrent chemotherapy with radiation (xeloda/cetuximab, cis/5fu, bevacizumab with FOLFOX or CAPOX) pre-treatment CEA level
A number of studies have investigated the value of pre-treatment CEA (pre-CEA) levels as response predictors in patients with rectal cancer receiving neo-CRT Das et al. reported that the CEA levels (cut-off value: 2.5 ng/ml) significantly predicted pCR in univariate analysis, but not in multivariate analysis [21, 28, 29].
T he Glasgow prognostic score serves as a prognostic factor, In the present study, there was a significant relation between pathological response and GPS 100% of patient achieved pCR were of GPS 0 with p <0.001 in agreement with Pathak, S et al., and McMillan, Donald C who reported that an elevated preoperative CRP was associated with poorer outcome compared with a normal preoperative level Buijsen et. al. claimed that the level of plasma CRP prior to treatment was a predictor of the tumour response following nRCT; however, our study did not find a significant correlation between the pre-treatment CRP level and a complete or good response following nRCT, which may have been due to the small number of participants [29 30, 31, 32]. The 3-year OS was 96% in arm 1 versus 92% in arm 2 which was statistically insignificant p=0.564 which in line with Markovina et al., who reported that 3-year OS was not different between the study nTNT cohort and the control NCRT cohort (96% vs 88%, P=0.67 [24].
In a Polish study, patients who had SCR plus consolidation chemotherapy had a better overall survival rate. However, the cumulative incidence of death in patients with tumour relapse was lower in the group treated with SCR and consolidation chemotherapy than in the group treated with long-course radiation and concomitant chemotherapy (23% versus 31%), despite the fact that the cumulative rates of local failure, distant failure, and death from non-cancer-related causes were similar between groups at 3 years. Uncertainty over the causes of the disparities led the authors to suggest that further investigation was required to resolve the problem [9].
Three-year OS was significantly related to pre-treatment CEA level (p=0.001), pre-treatment GPS (p=0.001) and tumour regression grade (p=0.001) but no significant differences were found as regard age, sex, performance, pathological stage, grade and Kras/Nras and BRAF mutation
92% of patients in arm 1, 88% in arm 2 achieved 3-year DFS which was statistically insignificant (p=0.627) different from Markovina et al., who reported that 3-year DFS in nTNT arm was 85% vs 68% in NCRT arm p= 0.032 this difference may be due to about 61.67% of patients in nTNT and 36% of patients in arm 2 omitted oxaloplatin in one or two cycles because of toxicity [24].
Three year DFS was significantly higher with GPS, preoperative CEA level and tumor regression grade (p<0.001, p<0.001, p<0.001 respectively but no significant difference were found as regard performance, sex, age, pathological stage and grad, Kras / Nras and BRAF mutation.
Conclusion
1. For Locally Advanced Rectal Cancer (LARC), neoadjuvant Chemo-Radiotherapy (CRT) followed by complete mesorectal excision is the standard treatment.
2. Short course radiotherapy followed by chemotherapy (near total neoadjuvant therapy) then TME offers comparable results in the form of response, tumour regression with similar toxicity, OS, and DFS.
3. Decreased pre-treatment GPS and CEA levels were associated with improved Tumour regression grade, overall survival, and DFS.
4. Watch and wait strategy may be an appropriate option in patients who develop complete pathological responses with sphincter preservation advantage.
5. More studies in the larger set of LARC patients with longer follow-up duration may provide support for the findings of the current investigation and confirm the efficacy and more tolerability of total neoadjuvant therapy and its impact on the response, OS and DFS and reduced late radiotherapy toxicity.
References
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev/Prz Gastroenterol. 2019; 14:89-103.
- Gonzalez SJ, Mejia de Grubb MC, Levine RS. Primary and secondary prevention of colorectal cancer: An evidence-based review. Fam. Med Community Health. 2017; 5:78-84.
- Veruttipong D, Soliman AS, Gilbert SF, Blachley TS, Hablas A, et al. Age distribution, polyps and rectal cancer in the Egyptian population-based cancer registry. World J Gastroenterol: WJG. 2012; 18:3997.
- Rokan Z, Simillis C, Kontovounisios C, Moran BJ, Tekkis P, et al. Systematic review of classification systems for locally recurrent rectal cancer. BJS open. 2021;5: zrab024.
- Papaccio F, Roselló S, Huerta M, Gambardella V, Tarazona N, et al. Neoadjuvant chemotherapy in locally advanced rectal cancer. Cancers. 2020 Dec 3; 12:3611.
- Pecori B, Lastoria S, Caracò C, Celentani M, Tatangelo F, et al. Sequential PET/CT with [18F]-FDG predicts pathological tumour response to preoperative short course radiotherapy with delayed surgery in patients with locally advanced rectal cancer using logistic regression analysis. PLoS One. 2017;12: e0169462.
- Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019; 25:4850.
- Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017; 18:336-346.
- Ciseł B, Pietrzak L, Michalski W, Wyrwicz L, Rutkowski A, et al. Long-course preoperative chemoradiation versus 5× 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019; 30:1298-1303.
- Valentini V, Van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, et al. Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother Oncol. 2015; 114:302-309.
- Benson AL. Epidemiology, disease progression, and economic burden of colorectal cancer. Journal of managed care pharmacy. 2007; 13:5-18.
- Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003; 16:376-388.
- Eren T, Burcu B, Tombalak E, Ozdemir T, Leblebici M, et al. Clinical significance of the Glasgow prognostic score for survival after colorectal cancer surgery. J Gastrointest Surg. 2016; 20:1231-1238.
- Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recur rence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012; 30:3827-3823.
- Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, et al. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget. 2016; 7:45513.
- Dindo D, Hahnloser D, Clavien PA. Quality assessment in surgery: riding a lame horse. Ann Surg. 2010; 251:766-771.
- Santos MD, Silva C, Rocha A, Matos E, Nogueira C, et al. Prognostic value of mandard and dworak tumor regression grading in rectal cancer: study of a single tertiary center. International Sch Res Not. 2014;2014.
- McLachlan SA, Fisher RJ, Zalcberg J, Solomon M, Burmeister B, et al. The impact on health-related quality of life in the first 12 months: A randomised comparison of preoperative short-course radiation versus long-course chemoradiation for T3 rectal cancer (Trans-Tasman Radiation Oncology Group Trial 01.04). Eur J Cancer. 2016; 55:15-26.
- Hazra A, Gogtay N. Biostatistics series module 7: the statistics of diagnostic tests. Indian J Dermatol. 2017;62:18.
- Fleming C, Vendrely V, Rullier E, Denost Q. Organ preservation in rectal cancer: review of contemporary management. Br J Surg. 2022.
- Kazi M, Sukumar V, Desouza A, Saklani A. State-of-the-art surgery for recurrent and locally advanced rectal cancers. Langenbeck's Arch Surg. 2021;406:1763-1774.
- Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst.2014;106:248.
- Song M, Li S, Wang H, Hu K, Wang F, et al. MRI radiomics independent of clinical baseline characteristics and neoadjuvant treatment modalities predicts response to neoadjuvant therapy in rectal cancer. Br J Cancer. 2022:1-9.
- Markovina S, Youssef F, Roy A, Aggarwal S, Khwaja S, et al. Improved metastasis-and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol *Biol* Phys. 2017;99:417-426.
- Chakrabarti D, Rajan S, Akhtar N, Qayoom S, Gupta S, et al. Short-course radiotherapy with consolidation chemotherapy versus conventionally fractionated long-course chemoradiotherapy for locally advanced rectal cancer: randomized clinical trial. Br J Surg. 2021;108:511-520.
- Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5× 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016; 27:834-842.
- Hospers G, Bahadoer RR, Dijkstra EA, van Etten B, Marijnen C, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol.
- Peng H, Wang C, Xiao W, Lin X, You K, et al. Analysis of Clinical characteristics to predict pathologic complete response for patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. J Cancer. 2018; 9:2687.
- Yang KL, Yang SH, Liang WY, Kuo YJ, Lin JK, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat. Oncol. 2013; 8:1-9.
- Okugawa Y, Shirai Y, Toiyama Y, Saigusa S, Hishida A, et al. Clinical burden of modified Glasgow prognostic scale in colorectal cancer. Anticancer Res. 2018; 38:1599-1610.
- Liu Y, He X, Pan J, Chen S, Wang L. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017; 7:1-9.
- Partl R, Lukasiak K, Thurner EM, Renner W, Stranzl-Lawatsch H, et al. The elevated pre-treatment C-reactive protein predicts poor prognosis in patients with locally advanced rectal cancer treated with neo-adjuvant radiochemotherapy. Diagnostics. 2020; 10:780.