Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 2
Micro RNA-145, -155 and -328 as biomarkers in breast cancer Iraqi women patients
Aseel S Mahmood1*, Shahlaa M Salih2 and Mahmood A Muhi32Department of Biology, College of Biotechnology, Al-Nahrain University, Baghdad, Iraq
3Department of Biology, Al-Farabi University College, Baghdad, Iraq
Aseel S Mahmood, Department of Biotechnology, College of Science, University of Baghdad, Baghdad, Iraq, Email: drahmed_ameer@hotmail.com
Received: 16-Nov-2023, Manuscript No. OAR-24-120715; , Pre QC No. OAR-24-120715 (PQ); Editor assigned: 20-Nov-2023, Pre QC No. OAR-24-120715 (PQ); Reviewed: 30-Nov-2023, QC No. OAR-24-120715; Revised: 22-Jan-2025, Manuscript No. OAR-24-120715 (R); Published: 29-Jan-2025
Abstract
Breast Cancer (BC) is the major issue in current therapy due to lack of sensitive and specific biomarker of tumor. It has been suggested by a number of studies that several miRNAs (miRNAs or miR) control human BC. Non-coding RNA molecules like miRNAs pave the way for several potential avenues of early cancer detection. Cancer risk has been associated with certain miRNA expression profiles. The goal of this study is to characterize the miRNA blood profiles of healthy controls and women in Iraq who have been diagnosed with breast cancer. Blood samples from 40 Breast Cancer (BC) patients and 40 healthy controls were analyzed utilizing real-time quantitative Polymerase Chain Reaction (qPCR) and the delta technique to determine the expression of 3 microRNAs (micro-328, micro-155, and micro-145). Micro-155 was found to be overexpressed, whereas micro-145 was shown to be down-regulated in patients. Expression of micro-328 has not altered much, as demonstrated by the fold change. Based on the findings, micro-155 and micro-145 expression levels have the potential to be used as important predictive indicators for BC patients and as interested biomarkers for future therapeutic interventions.
Keywords
Breast cancer; microRNA; Reverse Transcription; Real-Time PCR
Introduction
Cancer of the breast begins when a breast cell multiplies uncontrollably. The tumour is largely composed of these cells. Malignant (cancer) tumors are those whose cells invade neighbouring tissues or spread to other organs. Some breast cancers, known as lobular cancers, develop in the milk-producing glands themselves, whereas the vast majority, known as ductal cancers, begin in the tubes that carry milk to the breast. Parameters that elevate a probability woman of improving tumor of breast include her age, her gender, the presence of benign breast disease, a history of breast cancer, and her BRCA1 or BRCA2 gene mutation [1]. Aside from the most prevalent kind of BC, there are a few more. When breast cancer cells reach the lymph system or the blood, they can travel to other parts of the body and cause new tumors [2]. The use of early screening and detection methods, particularly when accompanied with appropriate therapeutic interventions, has promise for reducing breast cancer death rates. This observation is based on the occurrence of breast cancer cases that originated in Iraq [3]. Several microRNAs (miRNAs) have been found to be downregulated in several disorders, including cancer of breast (BC). The atypical expression of microRNAs (miRNAs) implicated in the oncogenic process provide a suitable avenue for investigating their functional significance in the progression of cancer [4]. miRNAs are the non-coding RNAs that have been extensively investigated in scientific research. This sequence exhibit the ability to bind to specific messenger RNAs (mRNAs) inside the 3'-Untranslated Regions (UTRs), when its size ranging from 18 to 25 nucleotides in length. Through this binding, they exert regulatory control on mRNA synthesis by either inhibiting translation or inducing degradation. MicroRNAs (miRNAs) are widely believed to exert regulatory control over a significant proportion, namely greater than one-third, of genes that coding protein. Consequently, they have appeared as significant contributors in a wide range of biological processes, including migration, differentiation, cell proliferation, apoptosis and angiogenesis [5]. miR-145 was initially hypothesized to exist due to its resemblance to a verified mouse miRNA, and then proved to be present in humans. Notably, within the context of colorectal cancer, miR-145 exhibits significantly reduced expression levels. Approximately 52.5% of microRNA (miRNA) genes have been related to the cancer improvement and advance [6]. The downregulation of miR-145 has been observed in several malignancies. Extensive research has demonstrated that miR-145 exerts its influence on several cellular processes, including cell cycle regulation, proliferation, apoptosis, and invasion, through the targeting of different oncogenes [7]. Elevated miR-155 expression in Breast Cancer (BC) has been associated with a high expression level and is correlated with certain tumour subgroups and increased death rates. miR-155 has a pivotal role in breast cancer. There is a substantial positive correlation between elevated levels of miR-155 and grade of increased tumor [8]. A novel group of microRNAs has been identified as non-coding RNAs, which are small RNA molecules that modulate the post-transcriptional regulation of genes of interest. These molecules have diverse expression profiles in particular tissues and cells, responding to specific stresses at discrete progressive phases. In general, their mechanism involves the pairing of bases with partly complementary sequences found in the 3'-Untranslated Region (3-UTR) transcript of an interest gene. This interaction leads to either cleavage of the mRNA or inhibition of translation, so leading in the negative regulation of interest gene [9]. Considering the availability of access to a specific site and the ABCG2 protein and mature miR-328 levels were harmfully associated with parental MCF-7 cells and drug-resistant, the ABCG2 has the potential to be effectively regulated by the human microRNA-328 (hsa-miR-328) [10]. Moreover, the findings of this investigation demonstrate that miR328 exerts its influence on the 3-UTR region in order to have a negative regulatory effect on the production of ABCG2 protein. This suggests that the reduction of ABCG2 expression mediated by miR-328 significantly enhances the susceptibility of cancer cells to drugs [11].
Materials and Methods
Subjects
A total of 40 females, presenting with different breast cancer stages, were analyzed by the oncologist at hospital of AI-Kadhimiya teaching in Baghdad between October 2021 and April 2022. Among these women, twenty were classified as being at an early stage (stage I and II), while the other twenty were classified as being at an advanced stage (stage III and IV). The study participants were selected from a demographic consisting of individuals between the ages of 18 and 55. This study included a cohort of forty healthy women who were matched in terms of age and served as control participants.
Methods
Prior to laparoscopy, 5 mL peripheral blood samples were collected in heparinized tubes from both control subjects and patients. These samples were then assessed using the Direct-zolTM RNA Mini-Prep kit (R2051, ZYMO RESEARCH/USA) to analyze the circulating miRNAs in plasma. MiRNAs were eluted in a volume of 20 μl of nuclease-free water. The Prime ScriptTM RT reagent Kit was utilised for the synthesis of cDNA in accordance with the directions provided by the makers. The reverse transcription process consisted of total RNA at a concentration of 100 ng/μl, a synthetic stem-loop primer at a volume of 0.5μl, nuclease-free water at a volume of 4.5 μl, and 20X prime script reaction buffer at a volume of 2 μl. The Reverse Transcription (RT) reaction was conducted at a temperature of 42°C for a duration of 15 minutes using a SaCycler-96 thermal cycler manufactured by SACACE in Italy. Subsequently, the reaction was heat-inactivated for a period of 1 minute at a temperature of 85°C and subsequently stored at a temperature of 4°C. A PCR reaction mixture of 20 μl was prepared using the KAPA SYBR® FAST Universal PCR master mix (KAPA, USA) according to the manufacturer's instructions for qPCR. To prepare the reaction mixture, 10 μl of 2X SYBR green PCR master mix, 4 μl of cDNA diluted at a ratio of 1:4, and 0.5 μl of each forward and reverse primer mix were combined. Subsequently, 5 μl of nuclease-free water was added to achieve a final volume of 20 μl. U6 microRNA (miRNA) was utilized as an endogenous regulator. To activate the polymerase, a quantitative Polymerase Chain Reaction (qPCR) was performed. The activation step involved incubating the reaction mixture at 95°C for a duration of 7 minutes. Subsequently, the amplification phase consisted of 45 cycles, each consisting of denaturation at 95°C for 10 seconds, followed by annealing and extension at 60°C for 1 minute. Ultimately, the study of the melting curve was conducted by utilizing the dissociation characteristic of double-stranded DNA across denaturing temperature cycles.
Statistical analysis
The analysis of data was carried out utilizing SPSS version 23, while the figures were generated using GraphPad Prism 6. The results were expressed using descriptive statistical metrics such as the standard deviation, mean and standard error. The independent specimen t-test was employed to examine disparities in means. A probability equal to or less than 0.05 was deemed to be of significance. The subsequent formulae ascertain the change of fold:
• ΔCT=CT of target gene-CT of U gene.
• ΔΔCT= ΔCT of each sample-average control ΔCT.
• Fold change=2-ΔΔCT
The control value was established as 1, and it was observed that samples with values more than 1 exhibited upregulation, whilst samples with values less than 1 exhibited downregulation.
Results
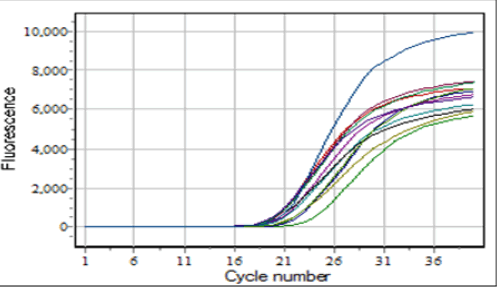
The gene expression levels of certain miRNAs were evaluated utilizing RT-PCR. This technique involved the use of a specific stem-loop primer to elongate the miRNA of interest, therefore turning the RNA into complementary DNA (cDNA). The estimation of folding was conducted utilizing the Livak approach, following the presentation of the resulting curves as seen in Figure 1 [12].
Fig.1. The RT-PCR curve findings.
The findings of the work indicate that the miRNA-145 expression was significantly decreased (0.307-fold change) in Breast Cancer (BC) patients in comparison with the controls (p=0.023). Conversely, mir-155 was found to be significantly up-regulated in BC patients in comparison with the controls (p=0.001). In contrast, the expression of MIR-328 exhibited statistically negligible alterations (p=0.09) when comparing patients to healthy controls (Table 1).
| miRNA | mean of ΔCt ± SE for patients | mean of ΔCt ± SE for controls | ΔΔCt | Folding | P value |
|---|---|---|---|---|---|
| miRNA-145 | 28.1 ± 1.12 | 26.4 ± 1.23 | 1.7 | 0.30779 | 0.023 |
| miRNA-155 | 21.7 ± 1.37 | 23.1 ± 0.26 | -1.4 | 2.63902 | 0.001 |
| miRNA-328 | 22.67 ± 2.4 | 22.43 ± 1.7 | 0.2 | 0.87055 | 0.093 |
Tab.1. Gene expression levels among patients and controls for all genes.
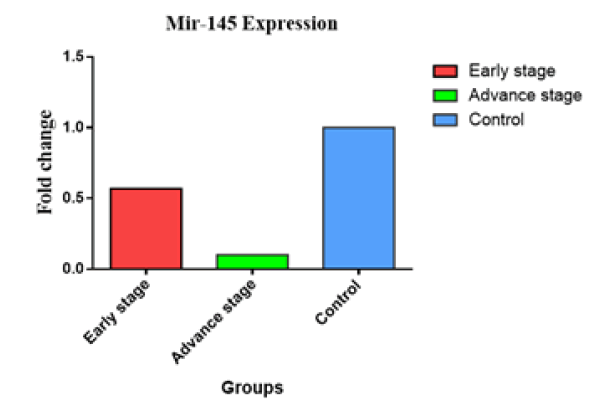
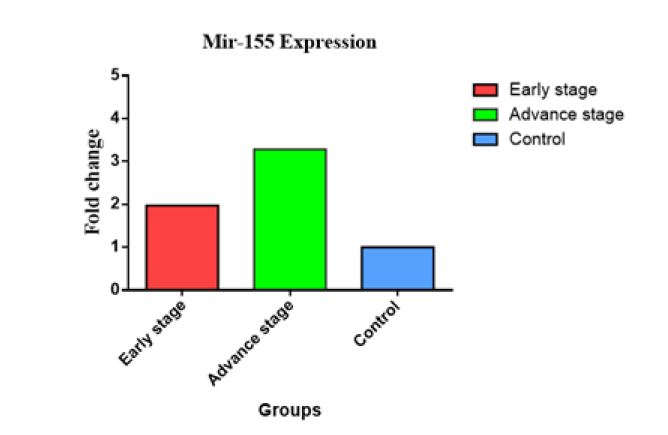
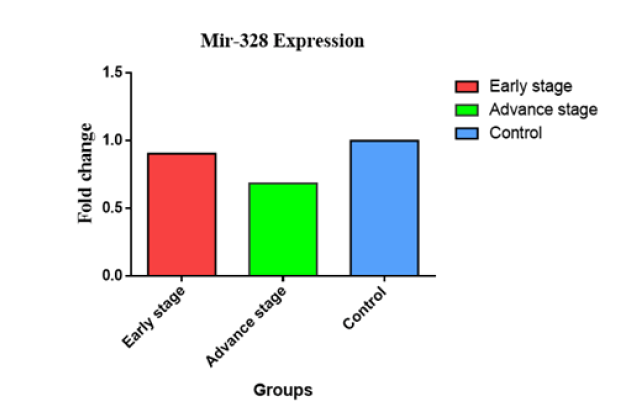
The Figure 2 presented in this study illustrates the relationship between the expression of the miR-145 gene and several clinicopathological criteria, such as tumor stages. The distribution of sample findings is visually represented utilizing a graph of bar. In individuals diagnosed with cancer in advanced-stage, the observed change of fold in level of miR-145 was significantly lesser (0.104 vs. 1) compared to those individuals diagnosed with early-stage cancer (0.564 vs. 1). The second figure. The Figure 3 illustrates the fold change of miR-155, indicating an miR-155 overexpression in later stages (3.282 vs. 1) in comparison with the early stages (1.974 vs. 1). In contrast, the examination of miR-328 gene expression levels across different stages revealed no statistically significant differences between early (0.687 vs. 1) and advanced (0.687 vs. 1) phases (Figure 4).
Fig.2. Comparison of miR-145 gene expression between early and late breast cancer.
Fig.3. Gene expression for miR-155 at early and advanced stages of breast cancer.
Fig.4. Expression of the miR-328 gene at its onset and progression through BC.
Discussion
Breast Cancer (BC) is a perilous ailment that exerts a substantial influence on the health and well-being of women. The initial neoplasm originates inside the mammary gland, however once acquiring invasiveness, it has the potential to disseminate to the lymph nodes located proximal to the breast [13]. Breast Cancer (BC) exhibited the highest incidence and death ratio across all the types of malignancies that were examined [12]. Breast cancer lacks distinct symptoms during its initial phases, resulting in delayed detection among a majority of patients. Consequently, this delay in diagnosis hinders timely intervention, thereby contributing to an unfavourable prognosis. The aetiology of breast cancer remains mostly ambiguous in contemporary times [14]. The identification of novel miRNAs elicits further scholarly attention towards the ongoing investigation. Since they are protected from RNases, it is often stable in blood serum and plasma [15], it is primarily involved in invasion apoptosis and proliferation of cell. It is highly conserved, cell-specific, and able to regulate migration, these processes [16]. Consequently, in clinical diagnostics, miRNA levels are employed as indicators and tumour biomarkers. The extracellular environment of blood arteries may include miRNAs released by tumour cells, or tumour cell death and lyses may be the source of miRNAs in circulating blood samples, as a result of these studies [17].
In this investigation, the expression of miRNAs 328, 155, and 145, was investigated in blood samples from female patients with BC who were in good health, as shown in Table 1. According to the study, patients with advanced stages had lower miR-145 levels than patients with early stages, and Figure 2 indicates that miR- 145 is down-regulated in BC. This is consistent with research on miR145 in ovarian and uterine cancer by Kim, et al. and Oksuz, et al., which suggests that miR145 may be have roles in the BC advance and development [18,19]. The expression of miR-145 may be correlated with the lymph node's depth of invasion, degree of differentiation, and severity of BC. Additionally, Sempere, et al. described that miR-145 is meaningly down-regulated in BC tissue in comparison with healthy tissue of breast based on northern blot and microarray examinations [20]. Similar to miR-205, miR-145 has reduced expression in tumors of breast relative to healthy breast tissue. Since changed miR-145 expression manifests early in BC, this miRNA may be used as a new biomarker for the early detection of malignancy. Since miR-145 may inhibit the production of ER-α mRNA and its coregulatory proteins, have a role in BC development, and prevent cells of BC from multiplying, it may be used as a tumor marker in BC diagnosis at the early stages. Conversely, the role of miR-145 in BC is yet unknown. Conversely, the miR-145 may stop BC from arising. Given that expression of miR-145 is down-regulated in BC and has been associated with a deprived prognosis, it is possible that miR-145 may soon be used as a significant prognostic marker for BC patients [21]. Despite the fact that miR-155 In contrast to patients in an earlier stage, the present work demonstrated MIR- 155 overexpression in advanced patients. Numerous research suggest that BC has higher levels of miR-155 expression, as Figure 3 illustrates. Only miR-155 has been discovered to be considerably raised in some investigations about BC, according to Iorio, et al., who also indicate that a high level of miR-155 is connected with a certain subtype of tumor and a high mortality rate [22]. These findings support the findings of the present investigation and imply that miR-155 has a major role in BC. Since other research has supported this finding, it was discovered that BC patients' blood levels of miR-155 were substantially higher than those of healthy controls [23]. Patients with BC exhibit greater levels of miR-155 than other groups, suggesting that this finding may have clinical predictive implications [24]. Patients with BC who express more miR-155 had higher tumour grades, more lymph node metastases, and advanced cancer stages, suggesting that miR- 155 may be employed as a clinical prognostic indicator [25]. The primary function of miRNAs is to bind to mRNAs' 30UTRs and suppress the mRNAs that they target, changing the actions of cells in the process. Therefore, identifying important miR-155 targets is necessary in order to determine BC and how miR-155 is involved in it [8]. It has been shown that miR-155, a potent apoptosis suppressor, functions as the onco-miR that targets caspase-3 [26]. In present investigation, it has been also shown that there was no discernible variation in the amount of MIR-328 gene expression between the later and earlier phases. The decreased miR RNA- 328 expression in BC implies this miRNA may be implicated in metastatic probable. Target site accessibility data suggest that miR- 328 can target the 3′-Untranslated Region (3′-UTR) of ABCG2 [11]. Figure 4 demonstrates a significant decrease in miR-328 expression across tumour and healthy tissue samples. Because GLUT1 is responsible for much of the increased glucose absorption rate in tumour tissues [27]. The decrease in the essential transport protein of glucose, GLUT1, mediated via miR-328 could put tumour survival in peril. Research suggests that miR-328 controls processes beyond only metabolism. MiR-328 regulates the BC resistance protein (ABCG2/BCRP) in human cancer cells, making them more sensitive to chemotherapy. Downregulation of miR- 328 has been linked to increased invasiveness and tumorigenicity in stem cells of cancer in vitro, according to earlier investigations [28]. Consistent with the current study, previous research suggests that reduced miR-328 gene expression may contribute to illness development. By down-regulating ABCG2 (the many drugs' molecular determinant, which pharmacokinetic characteristics in humans) in cells of MCF-7/MX100, miR-328 served an important organisational function. This resulted in increased sensitivity to mitoxantrone, which in turn had a profound effect on ABCG2 protein expression, miRNA abundance, and regulatory mechanisms. The expression of transporters including ABCG2/ BCRP, ABCC1/MRP1, and ABCB1/MDR1, which may be altered by miRNA, can influence cellular chemosensitivity and drug disposal. The expression or activity of miRNAs may affect the cellular defence against the medication. The most notable discovery is that ABCG2 overexpressing MCF-7/MX100 cells had lower expression of the ABCG2 regulating miR-328 than do ABCG2+ enriched cells [29,30]. Possible link between sickness and the consequent deficiency in these pathways.
Conclusion
The results showed that miR-155 is among the most significantly changed miRNAs in BC, suggesting that microRNAs in general have a role in the illness. Our findings suggest that miR-145 functions as a tumour suppressor. The downregulation of miR-145 in tumour tissues suggests it may be a promising novel target for diagnosis and therapy of breast cancer. Defects in microRNA-328, which plays a regulatory role, may contribute to illness development.
Acknowledgement
The authors would like to express their gratitude to Baghdad University in Baghdad, Iraq, and to all of the study's participants.
Conflicts of Interest
The authors declare that there is no conflict of interest
Funding
This paper was prepared, researched, written, and published independently, without the use of any external funding or financial donations.
Ethics Statement
This article was approved by the ethical committee of the college of science, University of Baghdad
Author Contribution
Author roles Aseel S. Mahmood: Design the review article Contributed to article writing and participation in molecular working methods. Shahlaa M. Salih: Contributed data and analysis tools and writing reviewer. Mahmood A. Muhi: Samples collection.
References
- Ali CA, Lafta FM, Al Sayyid MM, Al-Rekabi AA. BRCA1 gene expression is down regulated in both familial and sporadic breast cancer cases in Baghdad-Iraq. Iraqi J Sci. 2020; 61:34-41.
- Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019; 30:397-404.
[Crossref] [Google Scholar] [PubMed]
- AL-Ameri HD, Obaid IM, Shubber LA, Hussien AH. Barriers to Early Detection of Breast Cancer Among Iraqi Women in Baghdad. Univ Thi-Qar J Med. 2023; 25:115-125.
- Wang W, Luo YP. MicroRNAs in breast cancer: Oncogene and tumor suppressors with clinical potential. J Zhejiang Univ Sci B. 2015; 16:18-31.
[Crossref] [Google Scholar] [PubMed]
- Cui SY, Wang R, Chen LB. Micro RNAâ145: A potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med. 2014; 18:1913-1926.
[Crossref] [Google Scholar] [PubMed]
- Ahadi A. The significance of microRNA deregulation in colorectal cancer development and the clinical uses as a diagnostic and prognostic biomarker and therapeutic agent. Noncoding. RNA Res. 2020; 5:125-134.
[Crossref] [Google Scholar] [PubMed]
- Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010; 465:584-589.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Seo J, Nakamura T. Cellular approaches in investigating Argonaute2-dependent RNA silencing. Methods Mol Biol. 2018; 1680:205-215.
[Crossref] [Google Scholar] [PubMed]
- Pavlikova L, Seres M, Breier A, Sulova Z. The roles of microRNAs in cancer multidrug resistance. Cancers. 2022; 14:1090.
[Crossref] [Google Scholar] [PubMed]
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007; 39:1278-1284.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Xie W, Guan H. The diagnostic, prognostic role and molecular mechanism of miR-328 in human cancer. Biomed Pharmacother. 2023; 157:114031.
[Crossref] [Google Scholar] [PubMed]
- Alhashimy H, Abbas SA. Correlation between Trace Element Levels in Iraqi Breast Cancer Patients. Iraqi J Pharm Sci. 2023; 32:58-64.
- Sahan EJ. Evaluation of zinc, copper, and lead levels in the blood of breast cancer women in Baghdad City. Iraqi J Sci. 2022; 63:1-8.
- Jaafar NS, Hassan AF, Hamad MN. Evaluation of the Genotoxicity of the Aerial Parts of Iraqi Euphorbia cyathophora on Bone Marrow and Spleen Cells in Mice. Iraqi J Pharm Sci. 2023;32:41-45.
- Jin M, Xu Q, Li J, Xu S, Tang C. Micro-RNAs in human placenta: Tiny molecules, immense power. Molecules. 2022; 27:5943.
[Crossref] [Google Scholar] [PubMed]
- Jabbar A, Alshawi NN. Evaluation of The Effect of Fisetin against Cyclophosphamide-Induced Myelosuppression and Oxidative Stress in Male Albino Rats. Iraqi J Pharma. Sci. 2023;32:120-127.
- Heneghan HM, Miller N, Kelly R, Newell J, Kerin MJ. Systemic miRNA-195 differentiates breast cancer from other malignancies and is a potential biomarker for detecting noninvasive and early stage disease. Oncologist. 2010; 15:673-682.
[Crossref] [Google Scholar] [PubMed]
- Oksuz Z, Serin MS, Kaplan E, Dogen A, Tezcan S, et al. Serum microRNAs; miR-30c-5p, miR-223-3p, miR-302c-3p and miR-17-5p could be used as novel non-invasive biomarkers for HCV-positive cirrhosis and hepatocellular carcinoma. Mol Biol Rep. 2015; 42:713-720.
[Crossref] [Google Scholar] [PubMed]
- Kim TH, Song JY, Park H, Jeong JY, Kwon AY, et al. miR-145, targeting high-mobility group A2, is a powerful predictor of patient outcome in ovarian carcinoma. Cancer Lett. 2015; 356:937-945.
[Crossref] [Google Scholar] [PubMed]
- Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007; 67:11612-11620.
[Crossref] [Google Scholar] [PubMed]
- Quan Y, Huang X, Quan X. Expression of miRNAâ206 and miRNAâ145 in breast cancer and correlation with prognosis. Oncol Lett. 2018; 16:6638-6642.
[Crossref] [Google Scholar] [PubMed]
- Almohaywi M, Sugita BM, Centa A, Fonseca AS, Antunes VC, et al. Deregulated miRNA Expression in Triple-Negative Breast Cancer of Ancestral Genomic-Characterized Latina Patients. Int J Mol Sci. 2023; 24:13046.
[Crossref] [Google Scholar] [PubMed]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9:654-659.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Wu J. Role of miR-155 in breast cancer. Front Biosci. 2012; 17:2350-2355.
[Crossref] [Google Scholar] [PubMed]
- Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs: Potential as biomarkers and therapeutic targets for cancer. Genes. 2023; 14:1375.
[Crossref] [Google Scholar] [PubMed]
- Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007; 67:10782-10788.
[Crossref] [Google Scholar] [PubMed]
- Szablewski L. Glucose transporters as markers of diagnosis and prognosis in cancer diseases. Oncol. Rev. 2022; 16:1-12.
[Crossref] [Google Scholar] [PubMed]
- Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, et al. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer. 2012;106: 1320-1330.
[Crossref] [Google Scholar] [PubMed]
- Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, et al. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328,-519c and-520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011; 81: 783-792.
[Crossref] [Google Scholar] [PubMed]
- Mahmood A, Shafeq A, and Shafiq M. Uranium concentration variation dependency on gender correlated with age of bladder cancer patient. Int J Res Pharm Sci. 2019; 10:1730–1734.