Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 11
Evaluation of CTV-PTV margins for 3D-CRT of Head and Neck Cancers (HNC)
M.Najeh1,2*, Y. Benameur2, A. Marzak2, S. Sahraoui3, H. Jouhadi3, N. Benchakroun3, Z. Bouchbika3, T. Chekrine3 and N. Tawfiq32Medical Radiophysics Units (MRU), Mohammed VI Oncology Centre for Cancer ibn Rochd University Hospital, Casablanca, Morocco
3Department of Radiotherapy Mohammed VI Oncology Centre for Cancer Treatment, Ibn Rochd University Hospital, Casablanca, Morocco
M.Najeh, Laboratory of Genetics and Molecular Pathology (LGMP), Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Morocco, Email: mednajih11@gmail.com M.Najeh, Medical Radiophysics Units (MRU), Mohammed VI Oncology Centre for Cancer ibn Rochd University Hospital, Casablanca, Morocco, Email: mednajih11@gmail.com
Received: 21-Oct-2022, Manuscript No. OAR-22-77939; Accepted: 10-Nov-2022, Pre QC No. OAR-22-77939 (PQ); Editor assigned: 23-Oct-2022, Pre QC No. OAR-22-77939 (PQ); Reviewed: 06-Nov-2022, QC No. OAR-22-77939 (Q); Revised: 09-Nov-2022, Manuscript No. OAR-22-77939 (R); Published: 12-Nov-2022, DOI: 0
Abstract
Purpose: Patients movement and Organs mobility during Three Dimensional Conformational Radiotherapy (3D-CRT) may introduce errors especially during the treatment delivery, random and systematic errors are one of the most accurate errors. In this work we implanted a method to determinate the Clinical Target Volume (CTV) to Planning Target Volume (PTV) margins. The aim of this study is to reduce these errors probability by the evaluation of the optimal for CTV-PTV margin by studying the corrected values of deviations for HNC at the Mohammed VI cancer treatment centre treated between 2019 and 2021.
Material and Method: The study consists on creating a DATA base containing spatial movement of study group of 100 patients following the treatment machine axis Vrt, Lng, and Lat directions during the first four fractions. This study is done by Monaco (Version 5.11.02, Elekta, Stockholm Sweden) treatment planning system V 5.11.02 (TPS) and Mosaiq V 2.0 involving iViewGT R3.4.0 and XVI R5.0.3 on board imaging systems by Elekta Infinity Treatment Machine (Elekta Ltd, Crawley, UK). The patients were choosing randomly during the study and archived in a DATA base to collect the random and systematic errors, the root of the sum of the squares for the doubled value of method uncertainty based on the Van Herk and AL equation of PTV to CTV determination, Stroom’s model of Tumour Control Probability (TCP) and ICRU-50 formalism.
Included: The random choice was made to detect maximum of errors possible and to optimize the value of main function for errors margins obtained through the study. Each patient was controlled by Kv images (iViewGT R3.4.0 and XVI R5.0.3 on board imaging systems) to determinate the gap between the dosimetry–CT acquisition and the treatment session. The DATA analysis was made calculating about 400 parameters to obtain the main Function and standard deviation for every patient.
Results: CTV-PTV margin collected DATA was obtained by the correction values obtained by imaging system before treatment gave us a Mean (M) deviation function, the systematic errors (Σ) value and random errors (σ) respectively: 4.04 mm, 0.40 mm and 6.35 mm. the result obtained by the application of this values in the Van Herk equation for CTV-PTV margin was 5.7 mm.
Conclusion: The value calculated allowed us to have a validation of our quality treatment for three dimensional radiotherapy HNC and make sure that at Mohammed VI cancer treatment centre the norms and recommendations of radiotherapy treatments delivery are respected.
Keywords
3D-CRT, MONACO TPS, Head and Neck cancer, PTV, CTV
Introduction
In external beam radiotherapy, treatment planning of dose distribution is designed for every patient with the aim of having acceptable results of tumour control and normal-tissue toxicity. The target volumes consist of the Gross Tumour Volume (GTV) and the Clinical Target Volume (CTV), as defined by the International Commission on Radiation Units and Measurements (ICRU 2010) [1]. On the other hand, the Planning Target Volume (PTV) accounts for the various geometrical uncertainties and variations which limit the precision of delivering prescribed dose to tumour targets. It is well known that the PTV is formed by composing uniform margins around the CTV taking account of the possible setup errors, organs mobility and the patient’s motion during the treatment including the translational uncertainties. On the other hand, the entire PTV is given a prescription dose ensuring that the CTV receives the desired dose coverage during the treatment. However, Head and Neck Cancers (HNC) are one of the most complicated radiotherapy treatments due to the complex shape of the target volumes and the high dose delivered close to critical Organs at Risk (OAR). External beam radiotherapy is an advisable treatment of these type of cancers, therefore the patient’s setup before the treatment delivery is an important step due to the high precision required to reduce the introduction of irreparable treatment errors. Although there’s many unpredictable uncertainties included during the treatment setup and delivery representing a source of uncertainties that might cause random and systematic errors which are critical to the radiotherapy treatment success producing beams’ misalignment or leading to delivering radiation dose outside the target area.
The target volume delineation in such as cases is necessary and could be used to make sure that the Organs at Risk (OARs) are well protected during treatment delivery to reduce risk of further complications.
This local study aims to obtain a no uniform CTV-PTV margin caused by setup uncertainties based on a Van Herk statistical model considering translational errors and additional uncertainties for HNC patients to determinate the optimal clinical margins and protect the organs nearby to valid the respect of international recommendations for HNC 3D-CRT treatment [2-3]. Also by bringing light to this modality for centres with limited resources who are still using this technic to treat HNC, we are looking to evaluate this margins by taking prospective choice of patient treated by 3D-CRT following the international clinical recommendations at the Mohammed VI Oncology centre and introduce this margins to define the correct geometrical targeting uncertainties [4].
Materials and Methods
The study population consisted of a choice of 80 consecutive and randomized HNC treated patients in the Mohammed VI oncology centre between 2019 and 2021. These patients were accrued during the four first fractions to reduce each patient’s random errors and to keep the high precision of the setup realization. For the purpose of this study for assessing the time burden, the entire and delivery process was divided into specific steps (Table 1). Each step is a well-defined entity that allocated human resource based on prevalent departmental experience and practice to aid in the estimation of patients’ movement and the verification of deviations before treatment delivery
Tab. 1. Steps of the entire process of 3D-CRT and staff allocation
| Procedure (no. of staff) | Staff involved |
|---|---|
| Orfit making (3) | 1RO / 2 RTT |
| Planning CT (3) | 1 RO / 2RTT |
| Contouring approval /revision (1) | 1RO |
| Import/Planning (1) | 1 Physicist |
| Plan evaluation/approval/revision (1) | 1 Physicist / 1RO |
| Plan implementation (2) | 1 Physicist / 1RTT |
| Treatment | 2 RTTs / 1 RO |
The Patient's Immobolization
All the patients were immobilized following a supine position using three and five points thermoplastic mask keeping the neck rest and shoulder traction. Three staff members are involved in the process of immobilization before treatment (One Radiation Oncologist (one RO) and two Radiotherapy Technologist (RTTs)). Fiducials were placed after patient’s alignment to the scan-CT lasers and moved to the CT scanner for the planning CT scan.
Planning The CT-Scan and Treatment
The planning CT scan was done by immobilizing the patient on a flat couch six slices CT scanner (Optima CT-660, General Electric Medical Healthcare, Boston, Massachusetts, USA). High resolution axial images of the vertex to 10 cm below the carina were taken using 0.25 mm slice thickness and intravenous contrast product. Later on the collected data was transformed to a Monaco Sim TPS contouring station via the server network. Three staff members we re in vo lved in hte plan ning CT rpocess (one RO and 2 RTTs).
A scan-CT is attached to the patient as a reference for planning depending on the iso-centre location which is considered as the value of Immobilization during the treatment to have an identical position as the one the patient had during the CT-scan. Based on the ICRU-50 recommendation the Target volumes and the Organs-At-Risk (OARs) are define including the Gross Tumour Volume (GTV), a 5 mm clinical merging are adapted to define the Clinical Target Volume (CTV).
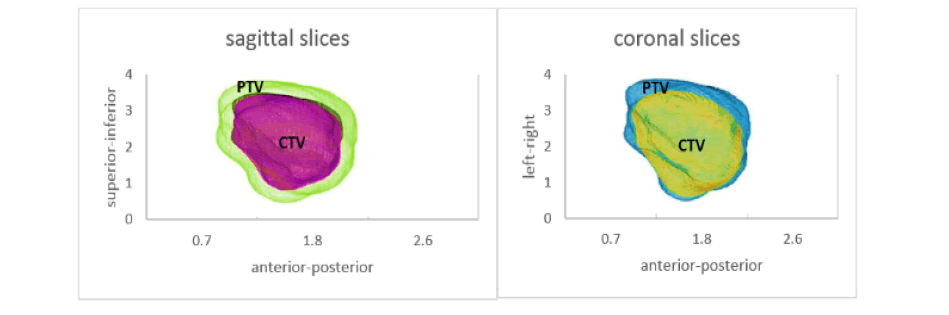
In this study following the ICRU-50 recommendations [1], 5 mm were applied to the CTV following the three axis of space as shown in the Figures 1 and 2 to define t h e pl a nning ta r get volume (PTV) otherwise defining t h e hi g h-risk and l o w -risk volumes in the neck due the high dose delivery depending on the characteristics and patterns of regional nodal metastasis. The prescription for phase I of 3D-CRT plan was 50 Gy delivered in 25 fractions. A sequential phase II for the gross disease to 66 Gy in 33 fraction (16 Gy in 8 fractions) then a phase III boost to the Tumour of 70 Gy in 35 fractions (4 Gy in two fractions) [5]. This p rocess u sually is c arried b y o ne R O and a pproved b y a senior RO before the planning began The 3D-CRT planning was carried out on Monaco TPS, configured for photons and electrons Multiple energies (6-MV and 18-MV for photons. 6-MeV, 8-MeV and 12-MeV for electrons) [6]. the Elekta Infinity machine used in this study is equipped with Multi Leaf Collimators (MLCs), iViewGT R3.4.0 and XVI R5.0.3 on board imaging systems. The treatment planning is done by the medical physicists including the preplanning and the validation of images importation and structure data set. The treatment planning was generated by using 6-MV photons with six to nine coplanar beams using MLC shaping based on the Beam’s Eye View (BEV) projection of the target volume as well as the OARs.
Figure 1: 3D Clinical 5 mm CTV-PTV margins simulation
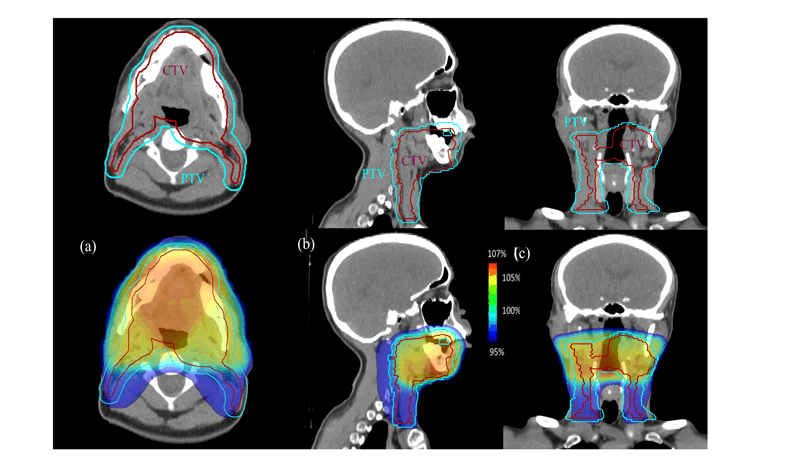
Figure 2: Dose distribution for 5 mm CTV-PTV margins of the 1st treatment phase (50Gy). The (a), (b) and (c) images are respectively the axial, coronal ad sagittal axis showing the CTV-PTV margins variation following these axes
The Treatment Verification and Set-up
The treatment was carried out on Elekta Infinity linear accelerator at a dose rate of 300 UM/Min. The treatment delivery is facilitated by using beams static mode with multi leafs conformation on the PTV. The machine is also equipped with IView and XVI imagery controlling systems to immobilize the patients and calculate the space difference of the first set up CT-scan immobilization. The calculations are made in the three space axis so the treatment delivery will be made with minimum margins errors possible, also by correcting these value and setting up the patient the right way, Kv Images are taking before every fraction to make sure the patients are set up the correct way. This process is managed by 2 RTTs and 1 RO [5-6].
Data acquisition and collection
For each patient we had to measure the vertical, horizontal and longitudinal variation (X, Y, Z) of the immobilization then calculate the mean function (m) of the errors to obtain the values of standard deviation (SD), systematic errors (∑), random errors (σ), main mean function (M) to obtain the final CTV-PTV margin using the Tumour Control Probability (TCP) model of tumours survival after irradiation in microscopic regions or possible extension. the Van Herk equation (i) [2-7] is one of the models that describes this probability of possible tumours extensions in microscopic regions, therefore the precision of having the right PTV to CTV margin is necessary to eliminate all tumour cells population.

Result
80 patients were included in the study, patients demographic are shown in Table 2. The data collection was done on Mosaiq system introduced by Elekta, we collected the special variation of each patient immobilization in the set-up for treatment process after the correction of set-up value to detect the random errors and systematic errors. As many of previous studies that has shown very optimal results, we had collected about 400 images using 2DKv images and 3D-CBCT in the three axis of space. Due to high risk of irradiation toxicity the 3D-CBCT were using sequentially. Applying the Van Herk equation to our collected values of immobilization to correct the spatial deviation due the patient mobility and set-up for each fraction. Our results were close to the ones introduced by the international recommendation of the ICRU formalism. The main Mean (M) function was 4.03 mm and the systematic errors (∑) and random errors (σ) are respectively equal to 0.52 mm and 6.35 mm. by converting this values in the Van Herk equation (1). Our results of PTV to CTV margins are 5.7 mm. However due to the proximity of many sensitive OARs and the high dose delivered for HNC the result obtained can be reduced in certain cases keeping an optimal dose coverage for the PTV and protection the healthy tissue and OARs in the area. We tried to show the quantities of devotions following the tree axis of space X, Y and Z to determinate on which axis the patient’s setup goes out of the CT-scan references before the treatment began and correct this values to obtain an optimal matching of position [3-9].
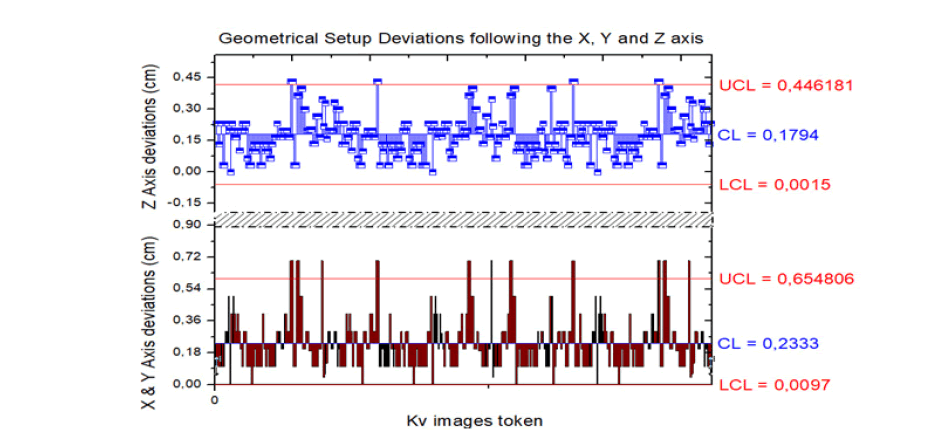
The results showed that patients deviate by a mean of 2.3 mm following the X horizontal axis, by a mean of 1.5 mm following the longitudinal Y axis and by a mean of 1.5 mm following the vertical axis of all patients. Mean deviations and standard deviations are represented in the Figure 3.
Figure 3: Patients’ Geometrical deviations from the treatment iso centre by Upper Control Limits (UCL), Control Limits (CL) and Lower Control Limits (LCL)
Discussion
While the technology transition in technics is taking place and a lot of centres are getting equipped with the latest treatment modalities and technics, on the other hand many other centres with limited resources are still using the 3D-CRT for treating HNC tumours and making sure that the quality if treatment delivered is adequate with the international recommendations. Although many local studies are made for CTV to PTV margins for this new technics and promoting the quality of treatment mentioning the advantages and inconvenient, the 3D-CRT was no more taken in consideration for such as studies. The implantation of 3D-CRT involves delivering conformal beams with high precision and whole tight CTV to PTV margins to the treatment planning process, right from the patient immobilization to accurate beam delivery and verification. Many factors at each stage will influence the selection of the margins applied to target volume depending on the patient’s morphology and immobilization each fraction [8]. We took the chance to make a local study about HNCs due the fast progression of tumours and how aggressive this type of cancer is, it was our priority to make sure that our patients’ treatment is done with a high precision and good setup for centres with limited resources and high activity level. Our collected data was collected by high precision while correcting the deviations before delivering the treatment fraction to make sure the target volume and our margins are respected. Due to the patients random and systematic errors that unpredictable the tolerance was Prometheus and we had very small deviations, otherwise all the deviation has been taking in consideration due to the high delivered dose and OARs placement. Theoretically we were assuming that the CTVPTV margin will be superior to 5 mm due to number of fractions and taking in count influence of systemic chemotherapy on the patient’s morphology.
Going through detailed data we found a statistically signification in the impact of the patient’s immobilization; systemic treatments such as chemotherapy, patient’s immobilization before the treatment, etc. these parameters are a main factor why we took minimum margins placed around a GTV to form the CTV as per ICRU-50 and 62, mainly ranging between 0.3 -1 cm according to the clinical case on the anatomical compartment within which the GTV is contained. In many studies the CTV to PTV margins are not surveyed, in our case following the ICRU-50 recommendations, ranging between 5-7 mm we took 5 mm as a mean margin for our patients willing to provide the OARs and keep a good dose distribution all over the target volume.
The survey was made for the four first fraction of patient’s immobilization taking spatial deviations and introduction a linear main function for each patient. The values were obtained on Mosaiq software with Kv and CBCT imaging, the selected images from these fraction were compared to the calculated reference images from the CT-scan 2D Scout to make sur the patient is setup the correct way following the CT-Scan setup maintaining the correction of deviations of the three space axis [9].
Conclusion
There is wide variation in the CTV to PTV margins depending on the patient’s target volume. In spite of ICRU recommendations, more effort should be done to define this margins. A local study is necessary for Radiotherapy centres to define this margins and try to reduce systematic and random errors. Based on this survey we have surmised that the variable impacting on the patient’s mobility increasing this error include:
i. Number of volumes delineated.
ii. Patient setup before treatment time.
iii. Time taken between the simulation day and treatment day.
iv. Patients morphology
Acknowledgments
We would like to the Mohammed VI oncology centre of Casablanca whole Radiotherapy staff for helping to answer subsequent queries and giving time to complete the study
Conflict of Interest
The authors declare no conflict of interests
References
- Prescribing IC. recording and reporting photon beam therapy
- Van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol * Biol* Phys.2000;47:1121-1135.[Google Scholar] [CrossRef]
- Van Herk M. Errors and margins in radiotherapy. Semin. Radiat Oncol 2004; 14: 52-64.[Google Scholar] [CrossRef]
- Van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol * Biol* Phys. 2002;52:1407-1422.
[Google Scholar] [CrossRef] - Biau J, Lapeyre M, Troussier I, Budach W, Giralt J, et al. Selection of lymph node target volumes for definitive head and neck radiation therapy: a 2019 Update. Radiother. Oncol.2019;134:1-9.
[Google Scholar] [CrossRef] - Grégoire V, Jeraj R, Lee JA, O’Sullivan B. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol. 2012 Jul 1;13(7):e292-300.
[Google Scholar] [CrossRef] - Stroom J, Gilhuijs K, Vieira S, Chen W, Salguero J, et al. Combined recipe for clinical target volume and planning target volume margins. Int J Radiat Oncol * Biol* Phys. 2014;88:708-714.[Google Scholar] [CrossRef]
- Stroom JC, De Boer HC, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol * Biol* Phys.1999;43:905-919.[Google Scholar] [CrossRef]
- Li H, Zhu XR, Zhang L, Dong L, Tung S, et al. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head-and-neck radiotherapy patients. Int J Radiat Oncol * Biol* Phys. 2008;71:916-925.[Google Scholar] [CrossRef]