Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 2
Evaluation of anticancer activity of Bacopa monnieri hexane fractions against breast cancer MCF7 cell lines (India)
Deepak Roshan VG, Mubashira KP, Sheethel KP, Surya Haritha S and Vipin Gopinath*Vipin Gopinath, Division of Genetics and Cytogenetic, Department of Clinical Laboratory Services and Translational Research, Malabar Cancer Centre, Thalassery, Kerala, India, Email: gopinath.vipin@gmail.com
Received: 30-Nov-2020 Accepted: 19-Jan-2021 Published: 18-Feb-2021
Abstract
Anticancer activity of Bacopa monnieri ethanolic extract was already reported against MCF7 and few other cancer cell lines. This study reveals the cytotoxic activity of hydrophobic hexane fraction of Bacopa monnieri against MCF7 breast cancer cell line. The hexane extract of B. monnieri was prepared using solvent extraction method and cytotoxic activity was assayed against MCF7 cell line. The IC50 values of hexane extract of B. monnieri was 53.0 μg/mL. The chloroform sub fraction of B. monnieri hexane extract doesn’t showed cytotoxic activity against MCF7 cells. The possible active molecules were identified using comparative LC/MS/MS analysis of parent bioactive hexane fraction with non-bioactive daughter chloroform fraction. The comparative LC/MS/MS analysis followed by data analysis using XCMS and METLIN library search identified 4406 unique features in hexane fraction. The cytotoxic activity in hexane fraction of B. monnieri might be due to bacoposide X and Merbarone.
Keywords
bacoposide x, merbarone, MCF-7 cells, phytochemical, natural product
Introduction
The cancer cases in India are increasing every year and it is the second most common cause of death in India. Lung, colon, prostate, and breast cancer account for more than half of the cancer deaths [1]. Although there are many therapeutic strategies, including chemotherapy, to treat cancer, high systemic toxicity, and drug resistance limit the successful outcomes in most cases. Accordingly, several new strategies are being developed to control and treat cancer [2]. The use of herbs as medicine is deeply rooted in human history and folklore and virtually in all human cultures. Plants have a long history of use in the treatment of cancer [3]. Plant-derived products are excellent sources for the discovery and development of new cancer chemotherapies. Natural products have been rediscovered as important tools for drug development despite in combinatorial chemistry [4].
Bacopa monnieri (L.) Wettst belonging to Scrophulariaceae family is a herbaceous plant traditionally commonly called as brahmi is used from time immemorial in Ayurvedic and folklore medicines. The various ethno medicinal uses mentioned are procognitive, antioxidant, anti-inflammatory, neuroprotective, and anticonvulsant activity [5]. In addition to these properties B. monnieri is a potential source of anticancer lead molecules, the anticancer activity of ethanolic fraction is already reported by Md. Nasar Mallick et al. [6]. In our study we are evaluating the anticancer property of B. monnieri hexane fraction against MCF-7 breast cancer cell line.
Materials and Methods
Chemicals
Roswell Park Memorial Institute (RPMI-1640), Fetal Calf Serum (FCS), and phosphate buffer saline were procured from Gibco, USA. The trypsin-ethylene diamine tetra acetic acid, penicillin-streptomycin solution, Dimethyl Sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich, USA. Trypan blue and MS calibration solutions were purchased from Thermo Fisher Scientific. All other solvents and chemical used were of LC/MS grade or hplc grade and procured from Merck India Ltd.
Plant material and extraction
The B. monnieri samples were collected from local area of Thalassery, Kerala, India. The extracts of B. monnieri was prepared using solvent extraction method and different fractions (hexane, chloroform and methanol) of whole plant extracts were prepared. The 5 mg powdered B. monnieri was mixed with 100 ml Hexane, chloroform or methanol and kept in magnetic stirrer overnight. The supernatant was taken and filtered using filter paper. At first the parent extracts of hexane, chloroform and methanol were used to screen for antitumor activity. Those extracts having bioactivity is subjected to sub-fractionation using solvents with adjacent polarity. Fifty ml hexane preparation was mixed with 50 ml of chloroform and kept of the magnetic stirrer and upper phase was taken and filtered using filter paper. All the extracts were passed through 0.22 µ membrane filter before using for in vitro activity on MCF-7cell lines. Similarly, DMSO control was also prepared and used in MCF-7 cell line. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay and trypan blue assay of all the extracts and of all fractions was carried out on MCF-7 cell line.
Cell viability and Cytotoxicity assay
The cells culture was trypsinated, and the cell count was adjusted to 1.0 × 105 cells/100 µL in RPMI-1640 containing 10% FCS. To each well of the 96-well microtiter plate, (approximately 100 µL media contain 10,000 cells) was added and kept it in the CO2 incubator overnight for adhering of cells on plate. Next day, the media was replaced with fresh complete media (100 µL). The drug (100 µL) was added in triplicate well (for one concentration). Then, serial dilution of the drug was done; again 100 µL of complete media was added to get the total volume of 200 µL (100 µL+100 µL). After completing the treatment, the 96-well microtiter plate was kept in a CO2 incubator. After 24 h study, 20 µL of MTT was added and left for 4 h in a CO2 incubator and after that the media was discarded from the wells carefully. Lysis buffer was added for dissolving the formazan crystals formed after addition of MTT, and left for 30 min in the CO2 incubator. Readings were taken at 570 nm at 24 h and 48 h. Percentage cytotoxicity of these extracts was calculated by using the formula: ([C-S]/C) × 100. Where, C is the absorbance of the control and S is the absorbance of the sample. Trypan blue cell viability assay was performed by automated cell counter Invitrogen Countess II FL instrument by Thermo Fisher Scientific, USA.
LC-MS/MS analysis
The LC/MS/MS analysis was performed using Thermo Q Exactive MS coupled with UHPLC system. The plant extracts were passed through 0.22 µ membrane filter before LC/MS/MS analysis. 0.1% formic acid was set as solvent A and 0.1% formic acid in acetonitrile was used as solvent B. The mobile phase was run in gradient mode with 5% of solvent B, at 8 minutes it raised to 40%, at 12 minutes to 70%, 100% at 15 minutes and maintained at 100% till 18 minutes. At 21 minutes the gradient was brought to 55%, at 25 reached 5% and maintained at 5% till 30th minute. The MS was calibrated in both positive and negative modes prior to the sample run based on vendor’s protocol. The run was made at positive ion mode with resolution set at 35000, AGC target at 3e6 and scan range of 80 m/z to 1200 m/z. The data acquisition and LC/MS/MS run was controlled by Thermo Xcalibur 4.1. Raw files were analysed using Thermo Mz vault and data normalization and downstream analysis was done using XCMS online platform [7]. The molecules were identified from the refined unique features by manual search with m/z values having three decimal point similarities in the METLIN database [8-10].
Results and Discussion
Cell viability and Cytotoxicity assay
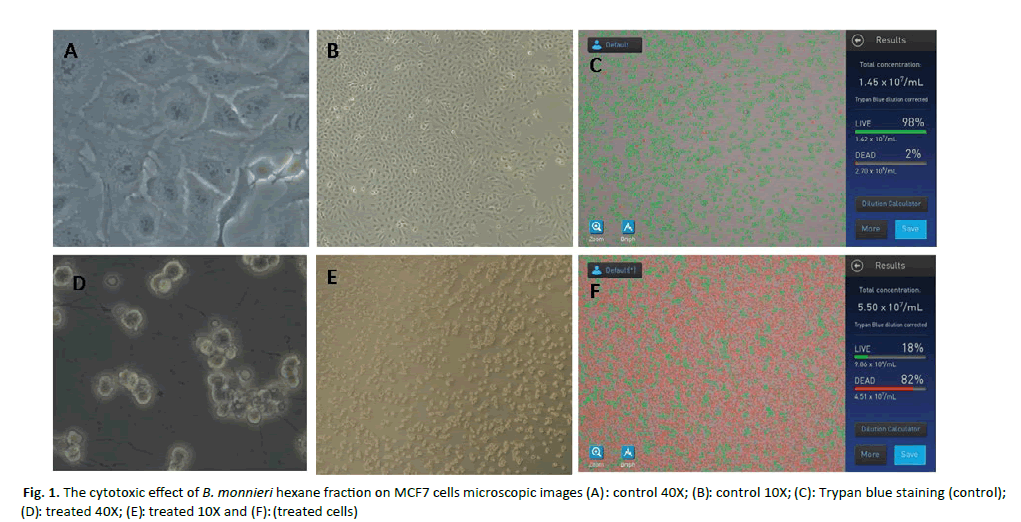
The B. monneiri fraction having highest cytotoxicity was the hexane fraction with an IC50 value of 53 µg/ml. Cell death of 82% identified in trypan blue staining and this result is concordant with MTT result of 20% cell viability on hexane fraction of B. monnieri. In order to understand the active principle behind the cytotoxicity of hexane fraction, the hexane fraction is again subjected to solvent extraction using lesser hydrophobic chloroform. The daughter chloroform fraction of hexane fraction doesn’t show any cytotoxic activity (Figure 1).
Figure 1: The cytotoxic effect of B. monnieri hexane fraction on MCF7 cells microscopic images (A): control 40X; (B) : treated 40X; (E): treated (treated cells) : control 10X; (D) 10X and (F): (C): Trypan blue staining (control);
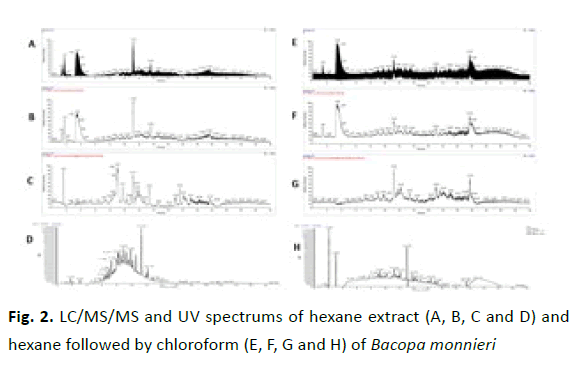
To find out the composition of different solvent extraction fractions of B. monnieri, we performed LC/MS/MS analysis on all the fractions. Figure 2 depicts the chromatogram of hexane fraction and chloroform soluble portion of the hexane fraction. The hexane fraction is the one with bioactive property while the daughter chloroform fraction was having no cytotoxicity against MCF7 breast cancer cells. Evidently these two chromatograms look very different, more peaks can be observed in hexane fraction and less peaks in the daughter chloroform fraction supporting the fact that not all the molecules are miscible in the chloroform and got separated out. Since the chloroform fraction doesn’t possess bioactive property the active molecule or molecules are those one which are immiscible in chloroform and present only in the hexane fraction.
Figure 2: LC/MS/MS and UV spectrums of hexane extract (A, B, C and D) and hexane followed by chloroform (E, F, G and H) of Bacopa monnieri
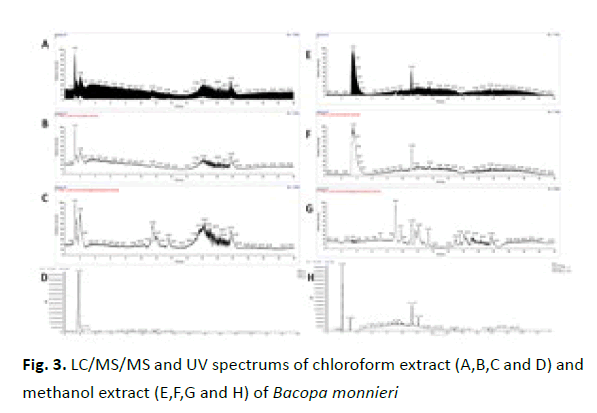
The chromatograms of chloroform and methanol fractions of B. monnieri are different from that of hexane fraction. They are very different from each other also, showing the efficacy of the extraction procedure to separate different composition of molecules from B. monnieri whole plant. Since the bioactive property is not present in the chloroform or methanol fraction much detailed analysis were not conducted on these fractions (Figure 3).
Figure 3: LC/MS/MS and UV spectrums of chloroform extract (A,B,C and D) and methanol extract (E,F,G and H) of Bacopa monnieri
The raw data files of B. monnieri hexane fraction run and chloroform daughter fraction run were subjected to comparative pair wise analysis in XCMS online platform. A total of 23809 features were identified altogether from both the samples. The results were shown as supplementary file 1. Out of this, 4406 features were found to be present only in the parent B. monnieri hexane fraction. The remaining 19403 features were present in both the hexane parent fraction and chloroform daughter fraction. In the analysis we get only very less features unique to daughter chloroform fraction and is absent to the parent hexane fraction, which indicate the stability of the identified features. The identified features were mapped into METLIN database using m/z values with three decimal points and zero δ ppm value. Those molecules identified from METLIN database consisted of the following molecules listed in Table 1. The table also shows the name chemical formula and structure retrieved from METLIN database.
Tab. 1. The list of representative identified compounds unique to the bioactive hexane fraction of Bacopa monnieri
| SL No | M/Z | Compound Name | Structure |
|---|---|---|---|
| 1 | 269.023 | 4-[(1,2,3,3,3-Pentafluoroprop-1-en-1-yl) oxy]benzoic acid - C10H5F5O3 | 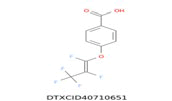 |
| 2 | 337.072 | 4(3H)-Quinazolinone,7-chloro-3-(3,5-dimethyl-4-hydroxyphenyl)-2-methyl – C17H15ClN2O2 | 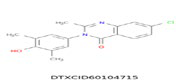 |
| 3 | 899.499 | Bacopaside X - C46H74O17 |  |
| 4 | 884.5002 | alpha-Solamarine - C45H73NO16 | 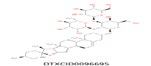 |
| 5 | 286.025 | Merbarone - C11H9N3O3S | 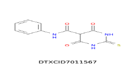 |
| 6 | 244.04 | 5,6-Dichloro-4-ethyl-1,4-dihydroquinazolin-2-amine- C10H11Cl2N3 | 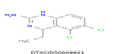 |
| 7 | 325.143 | Phaseollidin - C20H20O4 | 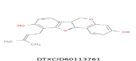 |
| 8 | 461.098 | N-(4-bromo-2-fluorophenyl)-6-methoxy-7-(piperidin-4-ylmethoxy)quinazolin-4-amine - C21H22BrFN4O2 | 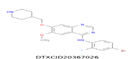 |
| 9 | 371.057 | 2,4-D 2-EHE - C16H22Cl2O3 |  |
| 10 | 394.173 | 1-[(2,5-dimethoxyphenyl)methyl]-4-[(3-nitrophenyl) methyl] piperazine -C20H25N3O4 |  |
| 11 | 912.505 | Saralasin - C42H65N13O10 |  |
| 12 | 339.0488 | Bromo(5-bromohexyl)hexylborane - C12H25BBr2 |  |
| 13 | 399.1278 | 3-(Morpholin-4-yl)-2-[(morpholin-4-yl)methyl]-1-phenylpropan-1-one--hydrogen bromide - C18H27BrN2O3 |  |
| 14 | 477.07 | 9,10-Anthracenedione,1,5-bis[(2-aminophenyl)thio]- C26H18N2O2S2 | 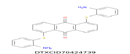 |
| 15 | 395.175 | Diethyl2,7-dichloro-4-methyl-2,7-dipropyloct-4-enedioate - C19H32Cl2O4 | 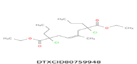 |
| 16 | 359.134 | Desmethyl fluoxetine - C16H16F3NO | 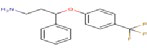 |
| 17 | 447.109 | [2-(Diphenylphosphorothioyl)ethyl](oxo)diphenyl-lambda~5~-phosphane- C26H24OP2S | 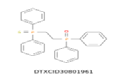 |
| 18 | 471.083 | 9,10-Dioxo-9,10-dihydroanthracene-1,5-diyldibenzoate -C28H16O6 | 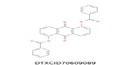 |
| 19 | 430.056 | cis-(1)-1-((2-(2,4-Dichlorophenyl)-4-((prop-2-ynyloxy)methyl)-1,3-dioxolan-2-yl)methyl)-1H-imidazoliumnitrate- C17H17Cl2N3O6 |  |
| 20 | 419.114 | Thiazolidine,3-(5-(4,6-dimethyl-2-quinolinyl)thiopentyl)-, dihydrochloride - C19H28Cl2N2S2 |  |
From the list of possible molecules having bioactive property, merbarone and bacoposide X are the most probable ones to provide cytotoxic property to the hexane fraction. This is the first report showing the existence of merbarone in B. monnieri. Bacoposide X is naturally occurring saponin present in B. monnieri. The mechanism behind the cytotoxicity of chloroform insoluble B. monnieri hexane fraction needed to be studied further.
Conclusion
This study identified cytotoxic activity in the hexane fraction of B. monnieri, whose active molecule is insoluble in the chloroform extraction. The activity of this fraction may be due to the molecules bacoposide X and Merbarone. Through this study we were able to create a natural product extraction process, having cytotoxic effect on breast cancer cell line MCF7. This fraction or these molecules can be used for future studies for creating novel chemotherapeutic options for breast cancer patients.
Acknowledgement
Deepak Roshan VG and Sheethel KP acknowledge the funding support from KSCSTE, YIPB program. All the authors acknowledge the Director, Malabar Cancer Centre for providing the facility to carry out the experiment.
References
- Programme NCR. Three year report of population-based cancer registries: 2009-2011. In: Indian Council of Medical Research, NCDIR-NCRP (ICMR) Bangalore; 2013.
- Taneja SC, Qazi GN. Bioactive molecules in medicinal plants: a perspective in their therapeutic action, in drug discovery and development. John Wiley and Sons. 2007;1-50.
- Patil A, Vadera K, Patil D, Phatak A, Juvekar, et al. In-vitro anticancer activity and phytochemical analysis of Bacopa monnieri (L.) Wettst. Int J Pharm Sci Res 2014;5:4432-4438.
- Khan MY, Aliabbas S, Kumar V, Rajkumar S. Recent advances in medicinal plant biotechnology. Indian J Biotechnol. 2009;8:9-22
- Koczurkiewicza P, jÅojewskib M, Piskaa K, Michalikc M, Wójcik-PszczoÅ?aa K, et al. Chemopreventive and anticancer activities of Bacopa monnieri extracted from artificial digestive juices. Nat Prod Commun. 2017;12:337-342
- Mallick N, Akhtar S, Najm Z, Tamboli ET, Ahmad S, et al. Evaluation of anticancer potential of Bacopa monnieri L. against MCF-7 and MDA-MB 231 cell line. J Pharm Bioall Sci. 2015;7:325-328.
- HuanT, Forsberg E, Rinehart D, Johnson CH, Ivanisevic J, et al. Systems biology guided by XCMS Online metabolomics. Nat Methods. 2017;14:461-462.
- Domingo-Almenara X, Montenegro-Burke JR, Ivanisevic J. XCMS-MRM and METLIN-MRM: a cloud library and public resource for targeted analysis of small molecules. Nat Methods. 2018;15:681-684.
- Guijas C, Warth B, Hermann G, Benton H, Palermo A, et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal Chem. 2018;90:3156-3164.
- Vijlder T, Laukens K. A tutorial in small molecule identification via electrospray ionizationâ?mass spectrometry: The practical art of structural elucidation. Mass Spectrom Rev. 2018;37:607-629.