Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 1
Estimation of late normal tissue complication for head and neck cancer patients treated with and without adaptive volumetric modulated arc therapy
Salam Abdulrazzaq Ibrahim Al-Rawi1*, Hassan Abouelenein2, Magdy Mohammed Khalil3, Haidar Hamza Alabdei4, Awf Abdulrahman Sulaiman1, Dalya Saad Al-Nuaimi1 and Mohamed El-Sayed EL Nagdy32Saudi German Hospital Cairo, Egypt
3Faculty of Science, Helwan University, Egypt
4College of Medicine, Baghdad University, Baghdad, Egypt
Salam Abdulrazzaq Ibrahim Al-Rawi, Baghdad Centre for Radiation Oncology and Nuclear Medicine, Medical City Complex, Ministry of Health/Environment, Baghdad, Iraq, Email: ahmedsalihdr2008@yahoo.com
Received: 01-Jan-2022, Manuscript No. M- 51562; Accepted: 24-Jan-2022, Pre QC No. P-51562; Editor assigned: 03-Jan-2022, Pre QC No. P-51562; Reviewed: 23-Jan-2022, QC No. Q-51562; Revised: 24-Jan-2022, Manuscript No. R-51562; Published: 30-Jan-2022
Abstract
Late radiation dose complications in patients with head and neck cancer treated with IMRT or VMAT represent a major problem; some of these complications came from the dose to organs that exceed their tolerance. In our study, patients underwent a new CT after ten and twenty treatment sessions and the initial plan then projected on the new CTs and the plans were called (hybrid plans). In hybrid plans, the dose for all organs was increased compared to initial plan (iplan) and in some cases the dose for organs was greater than their tolerance. The median maximum dose for spinal cord at iplan was 4113 [3967-4254] cGy and significantly increased (p<0.001) at Hplan1 to reach 4390[4154-4587] cGy and significantly increased again (p<0.001) at Hplan2. Also, the median maximum dose for brainstem at iplan was 5156[4561-5324] cGy then significantly increased (p<0.001) to 5321[4688-5545] cGy at Hplan1 and significantly increased again (p=0.001) to 5401[4821-5812] cGy at Hplan2. Other strategy was applied to maintain or decrease the dose to organs by make new plans with new dose constraints at session ten and twenty and called (adaptive plans). With adaptive plans we were able to maintain and reduced the dose for all organs (except for parotid glands). The median maximum dose for spinal cord was significantly reduced (p<0.00) at Aplan1 compared to iplan and another significant reduction at Aplan2 compared to Aplan1 were done (p<0.001). The median maximum dose for optic chiasm at iplan was 4471[863-5198] cGy and then decreased to 4481[740-5118] cGy at Aplan1 (p<0.001) and decreased again to reach 4228[741-5041] cGy (p=0.005) at Aplan2. So, with adaptive plan we were able to reduce dose to organs at risk an maintain the dose for organs below their tolerance and this will decrease the effect of late radiation toxicity complications for patients.
Keywords
head and neck cancer, VMAT, late radiation complications, adaptive plan
Introduction
Intensity modulated radiation therapy is the most common modality in the treatment of head and neck cancer with or without chemotherapy. And as result of high dose gradient that can be achieved by this modality, it provides a superior advantage over conventional radiotherapy in term of normal organ sparing. Although of the mentioned advantage, the steep gradient in dose could lead to an incidence of normal organs in the high dose region as a result of any small change in patient’s anatomy (weight loss for instance), and this could cause late tissue complications. Another important point that should be taken in account in the treatment of head and neck cancer is the large number of radio sensitive normal organs that surrounding the tumour [1]. Several studies showed the late effect of radiotherapy in head and neck cancer patients, where a study of 1544 patients with nasopharyngeal carcinoma treated with IMRT with median follow up more than 1 year, 0.13% of patients developed a brainstem necrosis after a time interval of 12.3 to 18.5 months [2]. Another study showed that a dose exceeds 50 Gy to brainstem leads to development of brainstem necrosis [3]. Also, a dose exceeds 54 Gy could cause limited risk of severe or permanent neurogical effect [4]. Despite of the caution that taken in treatment planning to ensure that the dose received to organs is below their tolerance, the change in patient’s anatomy during radiotherapy session may lead to variation between initial planned dose and actual received dose and cause the organs to receive dose higher than their tolerance. In this study, a dosimetric comparison between planned dose and dose received through radiotherapy session were achieved and its effect on late complication for number of organs. Also, a modification in treatment plan were done during radiotherapy session and compared to initial plan and its effect on late tissue complication.
Materials and Methods
The study includes 50 patients with different head and neck cancer sites, all patients treated with Volumetric Modulated Arc Therapy (VMAT) in concurrent with chemotherapy. Patients were undergoing contrast enhanced CT simulation using GE CT simulator (GE Revolution EVO, GE health care, Japan Corporation). The CT study set was performed with slice thickness of 2.5 mm. Contouring was performed by radiation oncologist using Monaco 5.1.1 treatment planning system. Also, PET imaging and Magnetic resonance imaging were acquired for all patients prior to CT simulation for better determination of tumour borders. VMAT planning was done for patients by a medical physicist with Simultaneous Integrated Boost (SIB). The prescribed dose was 69.96 Gy/33 fractions for Primary Target Volume (PTVP) and 60 Gy/33 fractions for high-risk Planning Target Volume (PTV60) and 54 Gy/33 fractions for low-risk Planning Target Volume (PTV54). The resulted plan then approved by the radiation oncologist by ensuring that the dose volume histograms for organs and targets are met with previous published dose constraints reports. After ten treatment fractions, a new contrast enhanced CT simulation was done, and new contouring achieved. The first plan (initial plan) was projected on the new CT with same planning parameters and at the same isocenter location. The isocenter was determined by placing radiopaque markers at first ten session isocenter location at the time of second CT acquisition. The projected plan was mentioned as hybrid plan (Hplan1). And the dose volume histogram for organs and targets was evaluated again. After another ten treatment fractions (20 fractions from the beginning of treatment), the above procedures were repeated and the new projected plan was mentioned as hybrid plan 2 (Hplan2). The dose for organs from initial 10 sessions and 10 hybrid plan1 sessions and 13 hybrid plan2 sessions was summed in one resultant plan called resultant hybrid plan (RHplan). The dose for each organ in resultant hybrid plan was calculated using the following equation:
D (a) RH=(D (a) IP/NIP)*nIP + (D (a) HP1/NHP1)*nHP1 + (D (a) HP2/NHP2)*nHP2
Where:
D (a) RH: the total dose received for the organ (a) at resultant hybrid plan.
D (a) IP: the total dose delivered to the organ (a) at initial plan.
D (a) HP1: the total dose delivered to the organ (a) at hybrid plan 1.
D (a) HP2: the total dose delivered to the organ (a) at hybrid plan 2.
NIP: total number of fractions of initial plan.
NHP1: total number of fractions of hybrid plan 1.
NHP2: total number of fractions of hybrid plan 2.
nIP: number of initial plan fractions received by the patient.
nHP1: number of hybrid plan1 fractions that received on the second CT.
nHP2: number of hybrid plan2 fractions that received on the third CT.
Also, new plan with new dose constraints was performed on the second and third CTs taking into account the anatomical changes happened to patients during radiotherapy sessions and theses plans were called adaptive plan 1 and adaptive plan 2 respectively. The dose for organs from initial 10 sessions and 10 adaptive plan1 sessions and 13 adaptive plan2 sessions was summed in one resultant plan called resultant adaptive plan (RAplan). The dose for each organ in resultant adaptive plan was calculated using the following equation:
D (a) RA = (D (a) IP/NIP)*nIP + (D (a) AP1/NAP1)*nAP1 + (D (a) AP2/NAP2)*nAP2
Where:
D (a) RA: the total dose received for the organ (a) at resultant adaptive plan.
D (a) IP: the total dose delivered to the organ (a) at initial plan.
D (a) AP1: the total dose delivered to the organ (a) at adaptive plan 1.
D (a) AP2: the total dose delivered to the organ (a) at adaptive plan 2.
NIP: total number of fractions of initial plan.
NAP1: total number of fractions of adaptive plan 1.
NAP2: total number of fractions of adaptive plan 2.
nIP: number of initial plan fractions received by the patient.
nAP1: number of adaptive plan1 fractions that received on the second CT.
nAP2: number of adaptive plan2 fractions that received on the third CT.
Finally, a dosimetric comparison for organs and targets between initial plan, hybrid plan and adaptive plan was performed and the late complications probability for patients as result from dose delivery from each plan was evaluated.
Results
In the hybrid plans there were significant changes in dose delivery to almost all organs if we compared them to dose of initial plan. Spinal cord showed significant increase in maximum dose value (p<0.001) at Hplan1 compared to iplan, where the median maximum dose for spinal cord at iplan was 4113[3967- 4254] cGy then increased to 4390[4154-4785] cGy at Hplan1. Spinal cord showed again a significant increase (p<0.001) in maximum dose delivery at Hplan2 with median maximum dose value 4598[4291-4959] cGy as it compared to Hplan1. In overall, the maximum dose for spinal cord at RHplan showed significant increase (p<0.001) if we compared it to its value at iplan, where the median maximum dose for spinal cord at RHplan was 4482[4210-4686] cGy. Taking another organ for dosimetric evaluation which is the brainstem, brainstem showed significant increase (p<0.001) in its maximum dose at Hplan1 compared to iplan. Where the median maximum dose for brainstem at iplan was 5156[4561-5324] cGy the value rose to 5321[4688-5545] cGy at Hplan1.
Also, the maximum dose for brainstem continued in its value increment to reach 5401[4851-5812] cGy at Hplan1, which is a significant increase to its value at iplan. In the end, the maximum dose for brainstem was significantly increased at RHplan compared to iplan. Where the maximum dose increased by 3.5% at RHplan compared to iplan. Table 1 is a summary of the dosimetric comparison of dose received to organs at risk at iplan, Hplan1, Hplan2 and RHplan.
According to clinical late toxicity studies as a result of radiotherapy, maximum dose equal or exceeds 50 Gy to brainstem leads to brainstem necrosis [3], and other study showed that a dose greater than 54 Gy will cause limited risk of severe or permanent neurogical effect [4], so with our study 36% of patients who continued in the same plan from the beginning to the end of sessions (hybrid plans) will face permanent neurogical effect. Besides, many studies revealed that maximum dose equal or greater than 50 Gy to spinal cord will exceeds the risk of myelopathy to 2% [5, 6], so with hybrid plans, 9% of patients will have 2% risk of myelopathy. Also, a maximum dose exceeds 55 Gy to optic nerves or optic chiasm will increase the chance of developing an optic neuropathy to 3% [7], in our study 15% of patients in hybrid plans the optic nerve received maximum dose greater than 55 Gy and in 22% of patients the optic chiasm received dose greater than 55 Gy. For mandible, a maximum dose equal or exceeds 70 Gy the patients will develop an osteoradionecrosis [8]. In hybrid plans 35% of patients will have the development of osteoradionecrosis.
Figure 1 showed organs at risk that received a dose exceeds their planned tolerance for hybrid plans.
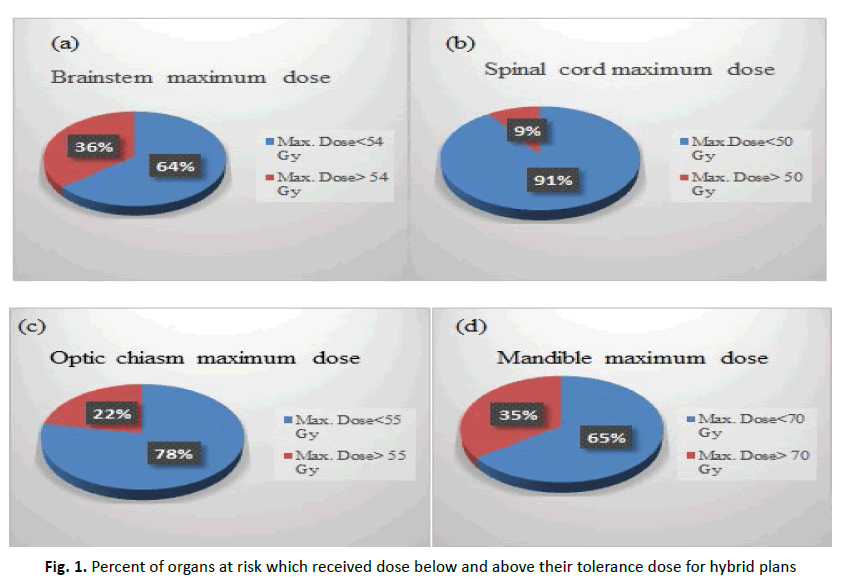
Figure 1: Percent of organs at risk which received dose below and above their tolerance dose for hybrid plans
On the other hand, the adaptive plans showed significant decrease in dose delivery to all organs except for parotid glands. Taking some examples for dose decrement for some organs, the mandible showed significant decrease in its maximum dose value at Aplan1 compared to iplan.
The median maximum dose for mandible at iplan was 6814[6500-6952] cGy then significantly decreased (p<0.001) to 6633[6286-6802] cGy at Aplan1. Then the maximum dose decreased again to 6600[6157-6781] cGy at Aplan2. So, the maximum dose of mandible at RAplan showed significant decrease (p<0.001) if it compared to iplan. Both lenses showed significant decrease in its maximum dose values at Aplan1 and Aplan2 compared to iplan. This is also applicable to eyes and optic nerves. Table 2 is a summary of the dosimetric comparison of dose received to organs at risk at iplan, Aplan1, Aplan2 and RAplan.
So, with adaptive planning, all organs received dose below their tolerance (or a decrement in number of patients with organs received dose exceeded their tolerance).
Figure 2 showed the percent of organs at risk that received dose below and above their tolerance in adaptive plans.
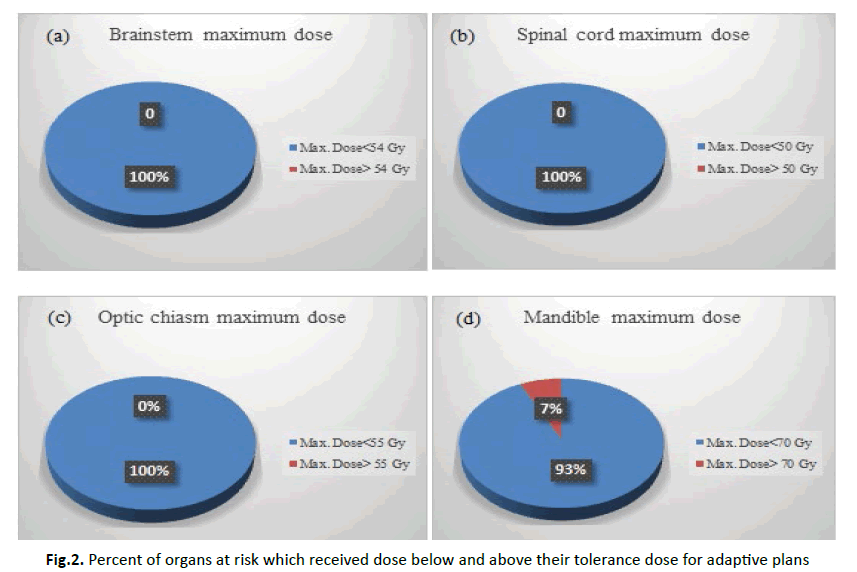
Figure 2: Percent of organs at risk which received dose below and above their tolerance dose for adaptive plans
Discussion
Modern radiotherapy techniques such as intensity modulated radiation therapy and volumetric modulated radiotherapy differs from conventional radiotherapy techniques in the ability of dose painting and sculpting the dose around healthy organs for better sparing.
IMRT and VMAT ability to cover the tumour and avoid nearby organs arises from the intensity modulation which enables to generate high dose gradient in very small area of tissue. But this steep dose gradient could be a double-edged sword, where any small change in patient anatomy like weight loss during radiotherapy sessions could leads to difference in dose distribution between planned dose and delivered dose, and a dose above dose tolerance may deliver to organs.
Researches showed that most patients face weight loss during radiotherapy sessions, Lonbro S et al, showed that head and neck patients who treated without using feeding tube during radiotherapy session had significant weight loss (4.7 ± 5.9) Kg [9].
Also, many other researches showed that patients with head and neck cancers underwent weight loss during the sessions of radiotherapy such as Vangelov [10], Dawson [11], Lee [12] and Ottosson [13].
The patient weight loss, leads to change in outer body contour, so this will cause an increment in dose delivery compared to planned dose (Figure 3). This can be explained as follows; the patient will have weight loss during RT sessions and this cause to change in outer body contour, so the distance between the skin and radiation source will increase, but the tissue thickness will decrease and radiation beam will face less attenuation during its travel through the body and in the end a higher dose will be received to the body (organs), that’s why almost all organs in our research showed significant increase in dose delivery (Table1).
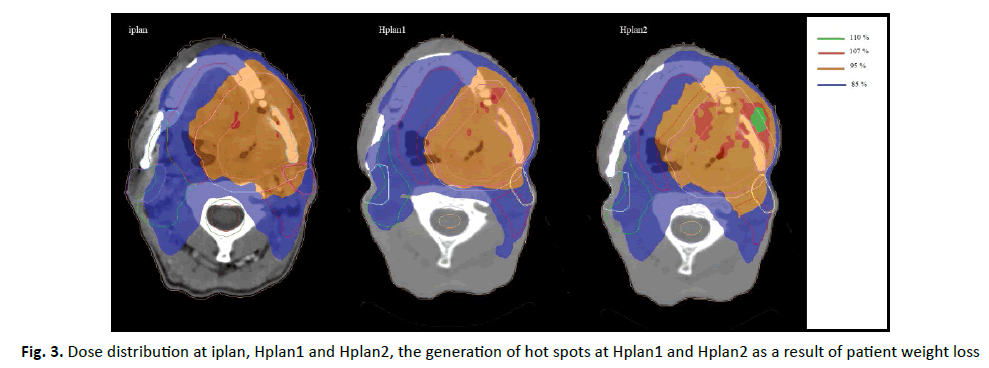
Figure 3: Dose distribution at iplan, Hplan1 and Hplan2, the generation of hot spots at Hplan1 and Hplan2 as a result of patient weight loss
| End points median[25th,75th] in cGy | iplan | Hplan1 | P value (iplan vs Hplan1) | Hplan2 | P value (Hplan1 vs. Hplan2) | RHplan | P value (iplan vs. RHplan) |
|---|---|---|---|---|---|---|---|
| Maximum dose to 0.03 cc of brainstem | 4985[4327-5181] | 5201[4459-5390] | <0.001 | 5322[4452-5617] | 0.001 | 5181[4478-5372] | <0.001 |
| Brainstem maximum dose | 5156[4561-5324] | 5321[4688-5545] | <0.001 | 5401[4821-5812] | 0.001 | 5342[4747-5516] | <0.001 |
| Maximum dose to 0.03 cc of spinal cord | 3999[3845-4115] | 4255[4018-4558] | <0.001 | 4496[4188-4812] | <0.001 | 4298[4058-4497] | <0.001 |
| Spinal cord maximum dose | 4113[3967-4254] | 4390[4154-4587] | <0.001 | 4598[4291-4959] | <0.001 | 4482[4210-5131] | <0.001 |
| Left eye maximum dose | 2952[603-4120] | 2951[531-4315] | 0.024 | 3218[446-4513] | <0.001 | 3031[545-4345] | 0.001 |
| Right eye maximum dose | 3490[486-4036] | 3654[433-4215] | 0.056 | 3698[395-4415] | <0.001 | 3633[437-4184] | 0.001 |
| Left lens maximum dose | 615[313-851] | 599[300-925] | <0.001 | 754[328-1119] | <0.001 | 653[297-952] | <0.001 |
| Right lens maximum dose | 486[190-840] | 498[190-919] | <0.001 | 542[188-1034] | <0.001 | 497[189-975] | <0.001 |
| Maximum dose to 0.03 cc of left optic nerve | 4015[444-5006] | 4118[480-5133] | <0.001 | 4385[506-5380] | <0.001 | 4192[481-5203] | <0.001 |
| Left optic nerve maximum dose | 4255[529-5187] | 4281[503-5381] | 0.006 | 4395[545-5588] | <0.001 | 4539[534-5391] | <0.001 |
| Maximum dose to 0.03 cc of right optic nerve | 4668[421-5030] | 4834[405-5220] | 0.009 | 5002[383-5534] | <0.001 | 4941[401-5284] | <0.001 |
| Right optic nerve maximum dose | 4936[453-5245] | 4990[437-5392] | 0.048 | 5108[420-5582] | <0.001 | 5081[435-5429] | <0.001 |
| Maximum dose to 0.03 cc of optic chiasm | 4265[728-5048] | 4196[785-5307] | 0.032 | 4877[784-5514] | <0.001 | 4539[786-5345] | <0.001 |
| Optic chiasm maximum dose | 4471[863-5198] | 4391[862-5479] | 0.015 | 4996[867-5624] | <0.001 | 4766[864-5427] | <0.001 |
| Mandible maximum dose | 6814[6500-6952] | 6805[6562-7121] | 0.021 | 6945[6562-7302] | 0.001 | 6841[6468-7174] | <0.001 |
| Left parotid mean dose | 2412[2124-2694] | 2625[2278-2884] | <0.001 | 3120[2567-3465] | <0.001 | 2721[2421-2905] | <0.001 |
| Right parotid mean dose | 2386[2205-2825] | 2564[2315-3013] | <0.001 | 2974[2631-3527] | <0.001 | 2634[2428-3074] | <0.001 |
Tab. 1. Dosimetric comparison for OARs at iplan, Hplan1, Hplan2 and RHplan
Another point that could cause a difference between initial plan and actual dose distribution, which is gross tumour shrinkage during RT sessions. Tumour shrinkage will lead to local density change (change in density in the volume nearby the tumour and surrounding tissue) and this will cause a change in dose deposition at this volume from what is in the initial plan.
Many researches showed that head and neck cancer patients will show tumour shrinkage through RT sessions. Lee H et al, studied the change in gross tumour volume during RT sessions for patients with nasopharyngeal carcinoma, they found that gross tumour volume is significantly reduced at the middle of RT session compared to initial gross tumour volume prior to RT session [14].
Other researches also showed that the gross tumour volume have been decreased significantly during radiotherapy sessions for head and neck cancer patients such as Haihua Yang [15] and Qiang Liu [16].
So, from the result in our research, keeping the same initial plan (iplan) during all radiotherapy sessions will cause an increase in dose delivery to almost all organs (that can be seen in hybrid plans) and for many patients the dose delivery was greater than their tolerance dose and this will lead to late radiation complications such as severe or permanent neurogical effect, brainstem necrosis and osteoradionecrosis.
The solution to reduce the late radiation toxicity complications is to take into account the change in patient’s anatomy; this could be done by make new plans every certain number of sessions (adaptive plans).
In our research we made an adaptive plan at session 10 and 20, and with adaptive plans we were able to significantly reduce (or maintain) the dose delivered to approximately all organs (Table 2) and this will decrease the late radiation toxicity complications for patients. The dose delivery to all organs was decreased in adaptive plan as it compared to initial plan except for parotid glands where the parotid glands showed increase in mean dose at adaptive plans as it compared to initial plan. The increase in dose for parotid glands is due to reduction in parotid gland volume during sessions. Many researches showed that parotid glands face a reduction in their volume during RT sessions [17-19]. Also, the increment in parotid mean dose is due weight loss which leads to parotid shift toward high dose region (region of tumour), which make the process of saving the parotid much harder with the adaptive plan.
| End points median[25th,75th] in cGy | iplan | Aplan1 | P value (iplan vs Aplan1) | Aplan2 | P value (Aplan1 vs. Aplan2) | RAplan | P value (iplan vs. RAplan) |
|---|---|---|---|---|---|---|---|
| Maximum dose to 0.03 cc of brainstem | 4985[4327-5181] | 4712[4219-4980] | <0.001 | 4631[4013-4901] | <0.001 | 4792[4160-5006] | <0.001 |
| Brainstem maximum dose | 5156[4561-5324] | 4934[4385-5135] | <0.001 | 4735[4215-5004] | <0.001 | 4940[4388-5123] | <0.001 |
| Maximum dose to 0.03 cc of spinal cord | 3999[3845-4115] | 3722[3485-3952] | <0.001 | 3697[3287-3941] | <0.001 | 3748[3522-3968] | <0.001 |
| Spinal cord maximum dose | 4113[3967-4254] | 3845[3627-4061] | <0.001 | 3755[3348-4005] | <0.001 | 3845[3629-4040] | <0.001 |
| Left eye maximum dose | 2952[603-4120] | 2752[345-3852] | <0.001 | 2531[345-3631] | <0.001 | 2575[435-3875] | <0.001 |
| Right eye maximum dose | 3490[486-4036] | 3031{404-3773] | <0.001 | 2845[373-3624] | <0.001 | 3129[528-3807] | <0.001 |
| Left lens maximum dose | 615[313-851] | 560[312-654] | <0.001 | 524[305-638] | 0.006 | 571[304-713] | <0.001 |
| Right lens maximum dose | 486[190-840] | 432[181-674] | <0.001 | 418[188-625] | <0.001 | 500[186-722] | <0.001 |
| Maximum dose to 0.03 cc of left optic nerve | 4015[444-5006] | 4020[353-4844] | <0.001 | 4012[364-4754] | <0.001 | 4011[386-4854] | <0.001 |
| Left optic nerve maximum dose | 4255[529-5187] | 4340[411-4940] | <0.001 | 4140[381-4912] | <0.001 | 4157[443-5007] | <0.001 |
| Maximum dose to 0.03 cc of right optic nerve | 4668[421-5030] | 4506[385-4869] | <0.001 | 4415[364-4781] | 0.008 | 4562[370-4912] | <0.001 |
| Right optic nerve maximum dose | 4936[453-5245] | 4788[420-5075] | <0.001 | 4535[378-4863] | <0.001 | 4826[452-5068] | <0.001 |
| Maximum dose to 0.03 cc of optic chiasm | 4265[728-5048] | 4366[705-4971] | <0.001 | 4122[675-4833] | 0.001 | 4281[687-4943] | <0.001 |
| Optic chiasm maximum dose | 4471[863-5198] | 4481[740-5118] | <0.001 | 4228[741-5041] | 0.005 | 4457[773-5162] | <0.001 |
| Mandible maximum dose | 6814[6500-6952] | 6633[6286-6802] | <0.001 | 6600[6157-6781] | 0.038 | 6678[6298-6822] | <0.001 |
| Left parotid mean dose | 2412[2124-2694] | 2415[2121-2841] | 0.213 | 2610[2232-3150] | <0.001 | 2471[2205-2909] | 0.03 |
| Right parotid mean dose | 2386[2205-2825] | 2449[2194-2801] | 0.957 | 2630[2245-3021] | 0.006 | 2490[2249-2840] | 0.2 |
Tab. 2. Dosimetric comparison for OARs at iplan, Aplan1, Aplan2 and RAplan
Conclusion
Head and neck cancer patients who underwent IMRT or VMAT treatment, keeping the same plan during all radiotherapy session will cause late radiation toxicity complications for patients, a replannig (adaptive plans) during radiotherapy is necessary to reduce the late radiation toxicity.
References
- Brodin NP, Tome WA. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018;86:8-18.
- Li YC, Chen FP, Zhou GQ, Zhu JH, Hu J, et al. Incidence and dosimetric parameters for brainstem necrosis following intensity modulated radiotherapy in nasopharyngeal carcinoma. Oral Oncol. 2017;73:97-104.
- Hoppe BS, Stegman LD, Zelefsky MJ, Wolden SL, Rosenzweig KE, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting- the MSKCC experience. Int J Radiate Oncol Biol Phys. 2007; 67:691-702.
- Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36-S41.
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the center nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093-1112.
- Kirkpatrick JP, Van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42-S49.
- Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, et al. Use of normal tissue complication probability models in clinic. Int J Radiat Oncol Biol Phys. 2010; 76:S10-S19.
- Brodin NP, Tome WA. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018;86:8-18.
- Lonbro S, Petersen GB, Andersen JR, Johansen J. Prediction of critical weight loss during radiation treatment in head and neck cancer patients is dependent on BMI. Supp Care Cancer. 2016; 24:2101-2109.
- Vangelov B, Smee R. Clinical predictors for reactive tube feeding in patients with advanced oropharynx cancer receiving radiotherapy ± chemotherapy. Eur Arch Otorhinolaryngol. 2017;274:3741-3749.
- Dowson P, Taylor A, Bragg A. Exploration of risk factors for weight loss in head and neck cancer patients. J Radio Prac. 2015;14:343-352.
Google Scholar Cross Ref
- Lee S, Wang T, Chu P. Prediction of weight loss during and after radiotherapy in patients with head and neck cancer: A longitudinal study. Euro J Oncol Nurs. 2019; 39:98-104.
- Ottosson S, Soderstrom K, Kjellen E, Nilsson P, Zackrisson B, et al. Weight and body mass index in relation to irradiated volume and to overall survival on patients with oropharyngeal cancer: a retrospective cohort study. Radia Oncol. 2014;9:160.
- Lee H, Ahn YC, Oh D, Nam H, Noh J M, et al. Tumour volume reduction rate during adaptive radiation therapy as a prognosticator for nasopharyngeal cancer. Canc Res Treat. 2016;48:537-545.
- Yang H, Tu Y, Wang W, Hu W, Ding W, et al. A comparison of anatomical and Dosimetric variation in the 15 fractions, and between fractions 16 and 25, of intensity-modulated radiotherapy for nasopharyngeal carcinoma. J Appl Clin Med Phys. 2013; 6:1-13.
- Liu Q, Liang J, Zhou D, Krauss D J, Chen P Y, et al. Dosimetric evaluation of incorporated patient geometric variation into adaptive plan optimization through probabilistic treatment planning in head-and-neck cancers. Int J Rad Oncol Biol Phys. 2018;101:985-997.
- Sreejeev AT, Joseph D, Krishnan AS, Gupta S, Paricha R, et al. Serial assessment of parotid volume changes during radical chemotherapy of locally advanced head and neck cancer: its implications in practice of adaptive radiotherapy. Anna Oncol. 2020; 6:1351.
- Hunter K, Fernandes L, Vineberg K, McShan D, Antonuk A, et al. Parotid glands dose-effect relationships based on their actually delivered doses: implication for adaptive replanning in radiation therapy of head-and-neck cancer. Int J Radiat Oncol Biol phys. 2013;4:676-682.
- Fiorentino A, Caivano R, Metallo V, Chiumento C, Cozzolino M, et al. Parotid gland volumetric change during intensity-modulated radiotherapy in head and neck cancer. Brit J Radio. 2012;85;1415-1419.



