Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 5
Enhanced phototherapeutic effects of laser-irradiated Ag NPsdecorated TiO2 Nanotubes in colon cancer cells
Maher I. Al-Shemri1, S. Z. Mortazavi1,2*, M. R. Khanlary1, Rana A. Ghaleb3 and A. Reyhani12Department of Physics and Energy Engineering, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran
3Department of Anatomy and Histology, University of Babylon, Babylon, Iraq
S. Z. Mortazavi, Department of Physics and Energy Engineering, Amirkabir University of Technology, P.O. Box 15875-4413, Tehran, Iran, Email: z.mortazavi@sci.ikiu.ac.ir
Received: 02-May-2025, Manuscript No. OAR-25-165351; , Pre QC No. P-165351; Editor assigned: 03-May-2025, Pre QC No. P-165351; Reviewed: 17-May-2025, QC No. Q-165351; Revised: 22-May-2025, Manuscript No. R-165351; Published: 29-May-2025
Abstract
Silver nanoparticles (Ag NPs) successfully decorated on titanium dioxide (TiO2 ) nanotubes (NTs). The effect of Ag NPs decorated TiO2 NTs on colon cancer cells (SW480 cells) is investigated with/without laser exposure at 532 and 808 nm in different times and various concentrations (15.6, 31.25, 62.5, 125, 250, 500μg/ mL). The aims to determine different doses and effective concentrations of Ag NPs-decorated TiO2 for low-energy laser treatment. Colon cancer (SW480) cells y was conducted for the green laser (=532 nm, power=300 mW) and NIR laser rays (= 808 nm, power=150 mw). The untreated cells (control group) maintain a high level of viability across all concentrations of Ag NPs decorated TiO2 NTs. The results indicate that the treatment with Ag NPs-decorated TiO2 NTs and green laser significantly reduced the cancer cell viability in a dose-dependent manner. This study demonstrates the potential of Ag NPs-decorated TiO2 NTs as a novel nanomaterial for photothermal therapy of colon cancer. This approach has several advantages over conventional therapies, including minimal invasiveness, and reduced side effects.
Keywords
Ag NPs decorated TiO2 NTs; Colon cancer cells (SW480 cells); Green laser exposure (@532 nm); NIR laser exposure (@808 nm)
INTRODUCTION
Cancer is one of the biggest causes of death worldwide. This condition has recently become more widespread in many countries [1]. This has posed a daunting task to the scientists working on how to come up with a treatable for this condition to consider other therapeutic approaches needed. The best-known approach to the tumor is a surgical resection of the malignant tissue combined with chemotherapy and/or radiation [2]. Most of today’s therapies cause lots of harm to the body and the therapies have issues like multidrug resistance. Radiotherapy is one of the widely recognized treatment methods, the use of ionizing radiation in a bid to exterminate cancer cells. This has been related to improvements in radiotherapy technologies and the introduction of new applications. A powerful objective in the search for new therapeutic strategies is aimed at either sparing healthy tissue as much as possible or reducing the extent of damage to it. Fluorine and U-Russell are examples of radiosensitive agents that are found in cancer cells [3]. The main consequence is that radiations given to the tumor also affect the healthy tissue around it. Conventional therapies tend to cause serious complications because of their nonspecific nature [4]. Recently, targeted therapy was created to deliver drugs to cancer cells while having minimal effects on other cells. The mortality rate due to colon and rectal cancer or Colorectal Cancer (CRC) is the third highest internationally, affecting more than 1.9 million people every year [5]. Obesity, smoking, and low physical activity are considered the most crucial factors that may lead to CRC [4]. Genes and diet are also important in the development of CRC [6]. Inorganic nanoparticles improve drug delivery with controlled action, which is beneficial for treatment. They can be functionalized to bind specifically at cancer cells hence, enhancing treatment effects [4,7-9]. More recent and promising therapies, known as photothermal and photodynamic therapies, employ laser light to activate nanoparticles to destroy cancer cells while leaving surrounding healthy tissues unharmed [5].
In order to solve these problems, nanotechnology provides a solution. Bio therapists say that the development of therapeutic and diagnostic solutions can be made very accurate through the use of nanoparticles which are any material between 1 and 100 nanometers in size. Due to their size and characteristics, such particles can circulate in the blood, attach to the cell surface proteins, and bring some treatment right to the cancer cells. While traditional radiation therapy affects both healthy and cancerous cells in the targeted area it poses great harm to the former, has limited therapeutic value, and is highly toxic, this is not the case with newer molecular targeted therapies. Titanium oxide nanostructures, in particular, are rather popular because of their highly valuable, non-corrosive, and photocatalytic properties, as well as relatively low cost. These characteristics make them useful across a wide spectrum of product types from creams and paints and plastics to pharmacological applications such as drug delivery, cellular imaging, photodynamic therapy, and biosensing. The developments inspired by findings of their photocatalytic characteristics since the early 1980s have contributed to a creation of new solar power, water purification systems, and cancer cures that exhibit their value in environmental and biomedical science [10-12]. In general, the field of research concerning metal-oxide nanoparticles is of significant importance, as it has the potential to contribute to the resolution of two pressing global problems: the development of effective methods for cancer therapy and water treatment [13].
Titanium dioxide (TiO2) nanoparticles can be applied in various fields because of their unique electrical, optical, and photocatalytic properties but controversy arises from recent research on the biological effects of the material. These realities derive from differences in the properties of nanoparticles and biological systems. In response, there is tremendous work mainly dedicated to the improvement of the photocatalytic activity of TiO2 for various purposes, especially for the non-surgical treatment of cancer and bacterial disinfection [14,15].
Silver (Ag) is one of the most recent nanoparticles that is perhaps frequently applied as a contrast agent. This is because silver is a biocompatible material and has a high Z value within the physical structure. Research conducted on the transport efficiency of nanoparticles in a cell pointed out that when it comes to cancer cells, silver nanoparticles of sizes that do not exceed a hundred nanometers in diameter are capable of penetrating the malformed cell structure and settling within the cell [16,17]. It has been demonstrated that silver nanoparticles (particles measuring less than 100 nm) are capable of traversing the cell membrane of cancer cells and accumulating within them. The findings of the study indicate that radiation therapy involving the use of silver nanoparticles can extend the survival period of mice with breast tumors by a factor of four in comparison to conventional radiation therapy alone [18].
Additions that include such components as silver (Ag) have worked well in enhancing the performance of TiO2. Silver-decorated TiO2 nanoparticles have high photocatalytic activity under visible light because of factors such as low electron-hole recombination and SPR, which allow the production of ROS. These ROS are used for cancer cell ablation in cancer therapy or for killing microorganisms in antimicrobial applications. The article also reports that Ag-TiO2 exhibits bactericidal activity even in the dark owing to the variation in the electronic characteristics. In general, to investigate non-invasive treatment with these Ag-decorated TiO2 nanostructures, one may employ either dark conditions devoid of light irradiation or light irradiation conditions, which will be discussed subsequently.
Hence the medical, antimicrobial, and environmental applications of TiO2 nanoparticles provide an abundant possibility, but more investigation needs to be done in the enhancement of their identity and compatibility with biological application specific in cancer treatment and drugs delivery system. The aim of this study is to investigate the toxicity of Titanium dioxide (TiO2) Nanotube (NTs) anchored by silver nanoparticles and to evaluate their photothermal anticancer effect in Colon cancer cells by laser irradiation.
MATERIAL AND METHOD
The stipulated specific titanium oxide nanotubes utilized in this study were procured from HW Nanomaterials, a Chinese company. These nanoparticles were produced employing electrochemical anodization, a method that involves subjecting titanium oxide to an electric current. Silver-decorated TiO2 was prepared by coating silver on TiO2 nanotubes. Under these conditions, the inner and outer parts of the TiO2 nanotubes were coated with silver nanoparticles, which are henceforth labeled as Ag-decorated TiO2 nanotubes. In the present experimental work, various chemicals were used for cell growth and development and to investigate the survival time of cells. Accordingly, RPMI-1640 cell culture medium (Gibco, England) supplemented with 10% fetal bovine serum (Gibco, England) and 1%Penicillin /streptomycin solution (Pan-Biotech/Germany) were used.
Characterization
In order to ascertain the crystal structure of the samples, X-ray diffraction (XRD) analysis was conducted using Philips PW1730 system with Cu-Kα radiation. The morphology and the particle size of the samples were determined using Field Emission Scanning Electron Microscopy (FESEM) with the Sigma 300-HV produced by the German Zeiss company, and Transmission Electron Microscope (TEM) with Philips, CM120. Moreover, energy dispersive X-ray analysis (EDX) was carried out by Oxford instrument.
RESULTS AND DISCUSSION
Fig. 1. presents the X-ray diffraction (XRD) pattern of Ag-decorated TiO2 nanostructures, which exhibits four distinct and intense peaks, thereby confirming the high degree of crystallinity of the samples. According to the standard card 96-901-2962, the XRD peaks observed at 2θ of 38.15̊, 44.32̊, 64.57̊, and 77.58̊ are associated with the cubic Ag diffraction planes (020), (111), (022), and (222), respectively. Additionally, the standard card 00-038-0699 asserts that the remaining peaks in the diffraction pattern are associated with the monoclinic phase of titanium oxide, characterized by equal lattice parameters.
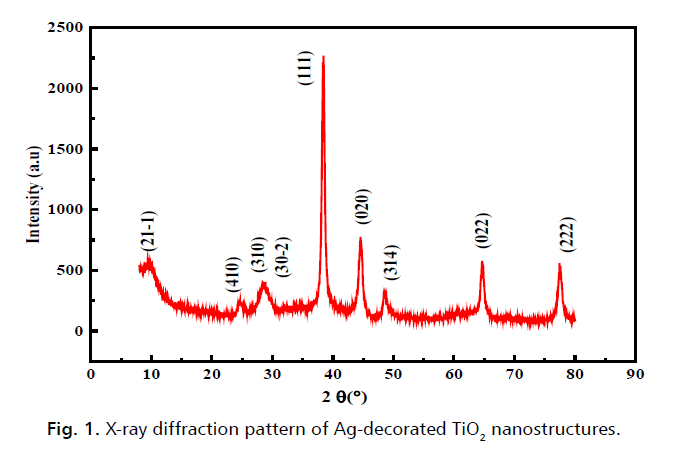
Figure 1: X-ray diffraction pattern of Ag-decorated TiO2 nanostructures.
As exhibited in Fig. 2a. and 2b., FESEM images of the synthesized Ag-decorated TiO2 nanostructure show a uniform nanotube morphology formed by the aggregation of spherical nanoparticles. Also, the Transmission Electron Microscope (TEM) images presented in Fig. 2c. and 2d. provide two magnifications of 50 nm and 100 nm, respectively, to provide more detailed information about the morphology of the nanostructure. As seen in these images, the nanostructure is composed of TiO2 nanotubes and spherical silver nanoparticles. As shown in Fig. 3., the average diameter of these spherical silver nanoparticles is 9.11 nm. However, a limited number of spherical silver nanoparticles with a diameter of close to 30 nm is also observed in the sample.
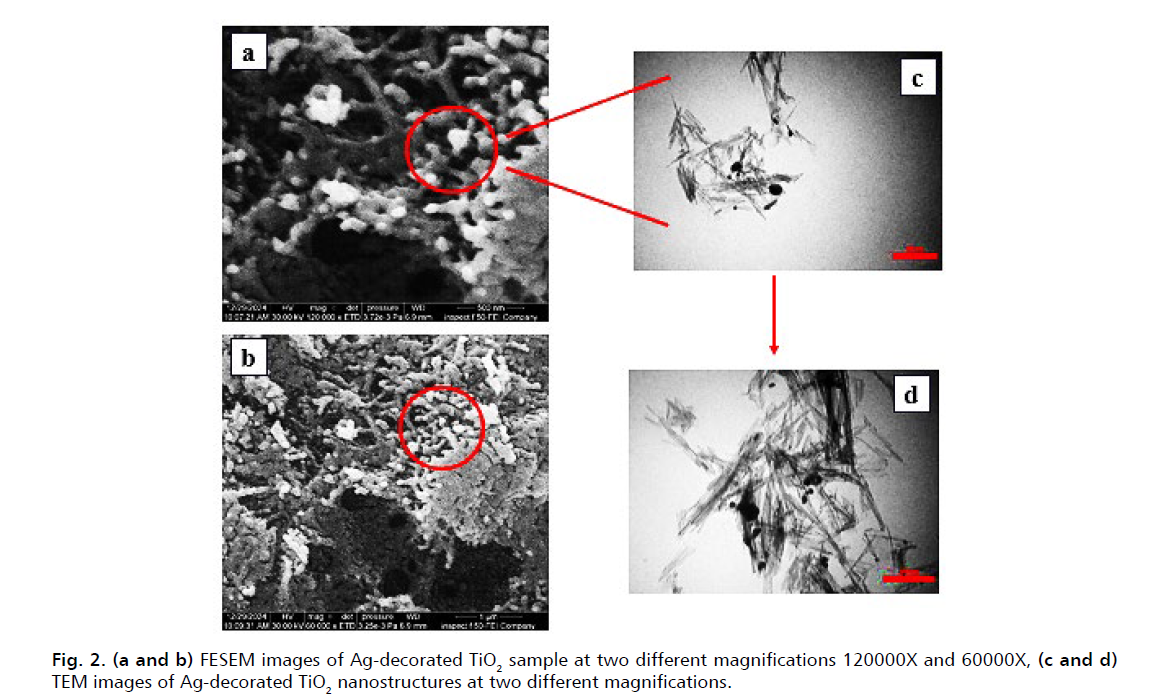
Figure 2: (a and b) FESEM images of Ag-decorated TiO2 sample at two different magnifications 120000X and 60000X, (c and d) TEM images of Ag-decorated TiO2 nanostructures at two different magnifications.
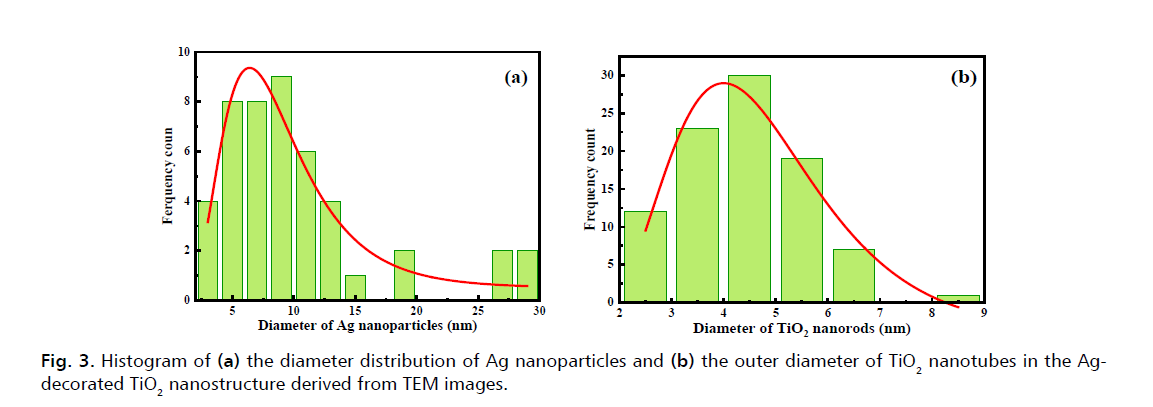
Figure 3: Histogram of (a) the diameter distribution of Ag nanoparticles and (b) the outer diameter of TiO2 nanotubes in the Agdecorated TiO2 nanostructure derived from TEM images.
The outer diameter of the TiO2 nanotubes and the diameter of the silver nanoparticles can be calculated using the TEM images and Digimizer software and fitting the Log-Normal function in Origin software on the bar graph of the particle size distribution (Equation 1) [19].
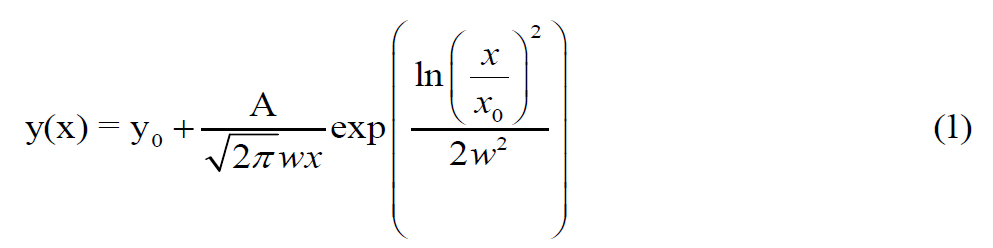
Where the diameter of the nanotubes (x) and their frequency counts (y) are the primary variables of interest. A, x, and w are additional parameters that are calculated through a process of fitting. The average diameter of the constituent nanoparticles and the diameter of the nanotubes are also obtained from the following Equation (Equation 2) [20]:
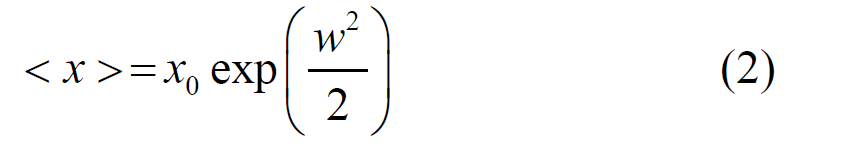
The histogram of the diameter of Ag spherical nanoparticles and the diameter of the TiO2 nanotubes obtained from TEM images is presented in Fig. 3a and 3b, respectively. The average of Ag nanoparticles and the average of outer diameter of TiO2 nanotubes estimated from TEM images are 9.11 nm and 4.8 nm, respectively.
Furthermore, the result of the energy dispersive X-ray (EDX) analysis of the sample is tabulated in Tab. 1. This indicates the presence of titanium, oxygen, and silver as the predominant chemical elements in the sample. The atomic percentage of silver in the sample is 7.35%, which verifies the successful decoration of Ag NPs on TiO2 nanotubes.
| Element | Line Type | k Ratio | Wt% | Wt% Sigma | Atomic % |
|---|---|---|---|---|---|
| O | K series | 0.01252 | 30.65 | 0.34 | 61.08 |
| Ti | K series | 0.10646 | 41.48 | 0.27 | 27.62 |
| Ag | L series | 0.05954 | 24.85 | 0.25 | 7.35 |
| Total | 96.98 | 96.05 |
Table 1. The corresponding EDX data of Ag-decorated TiO2 sample.
Reagents and solutions: According to Gibco's instructions, Phosphate Buffered Saline (PBS) was prepared by dissolving one PBS tablet in 500 mL of deionized distilled water and stirring continuously using a magnetic stirrer. PBS-solution was Sterilized by autoclaving and preserved sterile in a well-closed bottle until use. Then, Penicillin (10000 U/ml)-streptomycin (10mg/ml) solution was kept at 4 °C and added to the media at percent equal to 1%. For Trypsin-EDTA Solution, 1.2 g of Trypsin-EDTA powder was meticulously weighed using an analytical balance. The powder was then dissolved in 100 mL of deionized distilled water. The solution was meticulously prepared by gradually and continuously adding water and stirring it continuously at room temperature. The solution was filtered using graduated filters with 0.45 and 0.22 μm pore sizes it was sterilized by filtration.
Preparation of the culture medium
RPMI-1640 liquid medium was prepared from a powder form by dissolved according to the manufacture instruction (Gibco). The medium was prepared by dissolving 10.4 g of the powder in about 900 mL of deionized distilled water in a flask. Subsequent to this, the following components were added: 10 mL of antibiotic Penicillin/ Streptomycin solution and 2 g of sodium bicarbonate where/as appropriate. These components were added with constant stirring, globally. After that distilled water was added until the volume reached 1 L. In these circumstances, the pH of the culture medium was 7.4. The next step involved filter sterilization of the culture medium through the use of a graduated filter of diameter 0.45 μm and 0.22 μm respectively. After this procedure, the culture medium was incubated at the temperature of 37 °C before its use.
As previously explained, the serum culture medium was obtained by adding 10% Fetal Bovine Serum (FBS). Moreover, Colon cancer cells (SW480) were prepared in frozen tubes in the tissue culture laboratory of the College of Medicine/Babylon University. They were prepared according to the following steps:
Cell thawing: Frozen cells were thawed in a water bath set at 37 °C, washed with 70% ethanol, and inoculated into a 15 mL sterile culture plastic box containing the culture medium. The cells were then centrifugated at 800 rpm for 10 minutes, after which the obtained pellet was removed. The remaining cells were washed once and resuspended in a medium containing 10% FBS. They were subsequently transferred to a 25 cm2 tube and maintained at 37 °C. The medium was replaced the following day with a new medium. Culturing cell lines: This process employs trypsin to separate and categorize attached cells. Once confluence of a monolayer is obtained, the culture medium is removed and the cells are washed with warm PBS. A Trypsin EDTA solution is applied to the well and the cells are incubated and gently shaken to dissociate them. The cells are then cultured in a growth media containing 5-10% FBS as required for experiments with cell lines.
Colon cancer treatment
The required solutions and cancer cells, as well as cell culture lines, were prepared as described above. To investigate the toxicity of Ag NPs-decorated TiO2 nanostructures, a cellular toxicity test was conducted using laser treatment on a colon cancer cell line (SW480). The laboratory study aims to determine different doses and effective concentrations of Ag NPs-decorated TiO2 for low-energy laser treatment. As we mentioned before, tow types of lasers with different powers were used in this study, and each experiment, the best results were obtained for TiO2 and Ag NPs-decorated TiO2 nanotubes at concentrations (500, 250, 125, and 62.5 /mL) were obtained. Colon cancer (SW480) cells y was conducted for the green laser (=532 nm, power=300 mW) and NIR laser rays (=808 nm, power =150 mw). The TiO2 and Ag NPs-decorated TiO2 nanostructure were tested with Ag NPs colon cancer cells.
The impact of TiO2 and Ag NPs-decorated TiO2 nanostructures on cancer cells was examined after 24 hours incubation period. The calculation of viability% is based on the Equation (3) [21]:
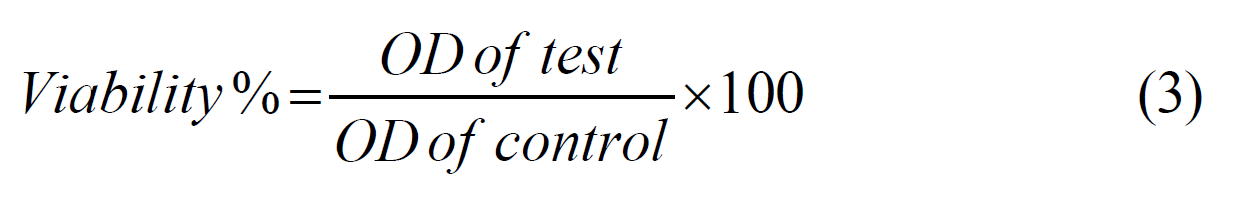
In this context, the OD of test represents the optical density of the treated sample, while the OD of control denotes the optical density of the untreated sample (control group). The 96 wellplates with a seeding density of 1×104 (cell/well) were used with the cancer cells for TiO2 and Ag NPs-decorated TiO2 nanostructures. These cells were exposed to 200 μL of six consecutive concentrations of each of the TiO2 nanotubes (15.6, 31.25, 62.5, 125, 250,500 μg/mL) (except for one column from each plates used as a control group that remained untreated for comparison. After completing this step, the dishes were covered with a plastic cover and incubated for 24 hours, and the growth of the cell line was evaluated by examining the cellular toxicity by MTT assay. Cytotoxicity can also be calculated by subtracting the percentage of viability from the value calculated with Equation 4, which is written according to Equation 5.
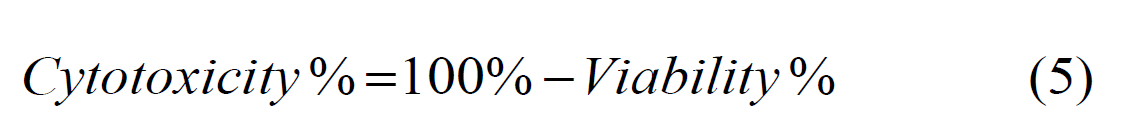
The effect of green laser at 532nm in TiO2 and Ag NPs-decorated TiO2 nanostructures on the colon cancer cell is investigated after 24 hours. The 96-well plates with a seeding density of 1×104 (cell/well) were used with colon cancer cells (SW480) and the wells of the plates were exposed to six different and consecutive concentrations (15.6,31.25,62.5,125, 250,500 μg/mL) from the TiO2 and Ag NPs-decorated TiO2 nanostructues were placed (except for one column from each plate as a control group). Each well was exposed to the green laser with a wavelength of 532 nm and a power of 300 mW at different times to create different radiation effects. Measuring the cytotoxic impact after 24 hours of incubation. The effect of laser treatment on the growth of the cell line was evaluated using the MTT cellular toxicity method.
Moreover, NIR laser with a wavelength of 808 nm was applied for more investigation with a 24-hour incubation period. The 96-well plates with a seeding density of 1 × 104 (cell/well) (as a control group) containing colon cancer cells (SW480), were treated with six different and consecutive concentrations of TiO2 and Ag NPs-decorated TiO2 (15.6, 31.25, 62.5, 125, 250, 500 μg/mL)). Each well was exposed to the NIR laser with a wavelength of 808 nm and a power of 150 mW at different times to create different radiation effects. Then the dishes were covered with plastic lids and incubated for 24 h. The effect of laser treatment on the growth of the cell line was evaluated using the MTT cellular toxicity method.
Fig. 4. shows the effect of silver nanoparticles (Ag NPs) decorated Titanium dioxide (TiO2) on colon cancer cells (SW480 cells). The untreated cells (control group) maintain a high level of viability across all concentrations of Ag NPs decorated TiO2, close to or at 100%. Cell viability in the treated cells shows a significant decrease as the concentration of Ag NPs-decorated TiO2 increases. As is clear the viability is almost negligible at the highest concentration. This suggests that Ag NPs-decorated TiO2 nanostructures has a strong inhibitory effect on the growth of SW480 cells, and its effectiveness increases with increasing concentration. This could potentially be a promising approach for colon cancer treatment. To discuss these findings in light of current scientific knowledge, recent studies, and literature on this topic show that silver nanoparticles (Ag NPs) have been found to have potential anticancer activities [22,23]. These nanoparticles can induce apoptosis and inhibit the proliferation of cancer cells, in the case of colon cancer cells, Ag NPs have been shown to decrease antiapoptotic genes and increase apoptotic genes [22] which leads to the induction of apoptosis, a form of programmed cell death, in the cancer cells [23]. So, it can be said that the experiment results align with the current scientific understanding and Ag NPs-decorated TiO2 can significantly reduce the viability of colon cancer cells, potentially offering a new avenue for cancer treatment.
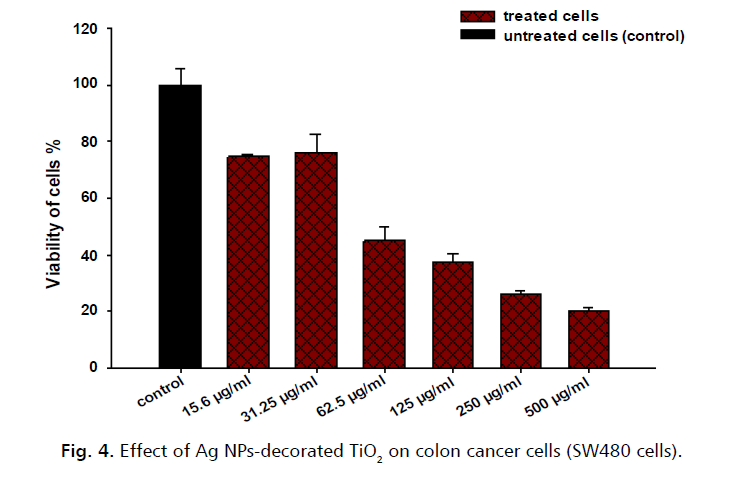
Figure 4: Effect of Ag NPs-decorated TiO2 on colon cancer cells (SW480 cells).
Furthermore, Fig. 5. presents the results of an experiment assessing the viability of SW480 colon cancer cells when treated with varying concentrations of Ag NPs-decorated TiO2 nearly all untreated cells (control) remained viable; at 15.6 µg/mL a slight reduction in cell viability is observed for treated cells; at 32.25 µg/mL there is not significant decrease in cell viability, indicating the effectiveness of the treatment; at 62.5 µg/mL further reduction in cell viability is noted, affirming a dose-dependent response. At higher concentrations of 125 µg/mL and 250 µg/mL, cell viability plummets drastically to below 20%. At the highest concentration tested, 500 µg/mL, almost all treated cells are non-viable. These results are comparable to the results of other studies conducted in this field [22-26]. In addition, Fig. 6. shows the results of an experiment that investigated the effect of silver nanoparticles (Ag NPs) decorated TiO2 on colon cancer cells (SW480 cells) after exposure to 150 mW of 808 nm infrared laser for 3 minutes. The viability of cells was measured by the MTT assay and compared between treated and untreated cells (control) at various concentrations of Ag NPs decorated TiO2. Results indicate that the Ag NPs-decorated TiO2 had a significant cytotoxic effect on the SW480 cells, as the cell viability decreased with increasing concentration of the nanomaterial. Although the control group showed approximately 100% cell viability, the treated groups showed 74.67%, 67.34%, 45%, 37.54%, 26.31% and 20.44% cell viability at 15.6, 31.25, 62.5, 125,250 and 500 µg/mL concentrations, respectively. This suggests that the Ag NPs-decorated TiO2 induced apoptosis (cell death) in the SW480 cells, especially at high concentrations. The mechanism of action of the Ag NPs-decorated TiO2 is likely related to the generation of Reactive Oxygen Species (ROS) and the photothermal effect [27]. The Ag NPs can act as photosensitizers, absorbing the infrared light and transferring the energy to the TiO2, which can produce ROS such as hydroxyl radicals and superoxide anions. ROS can damage the cellular components, such as DNA, lipids, and proteins, and trigger the apoptotic pathways. Moreover, the Ag NPs can also convert the light energy into heat, creating a localized high temperature that can cause thermal ablation of the cancer cells.
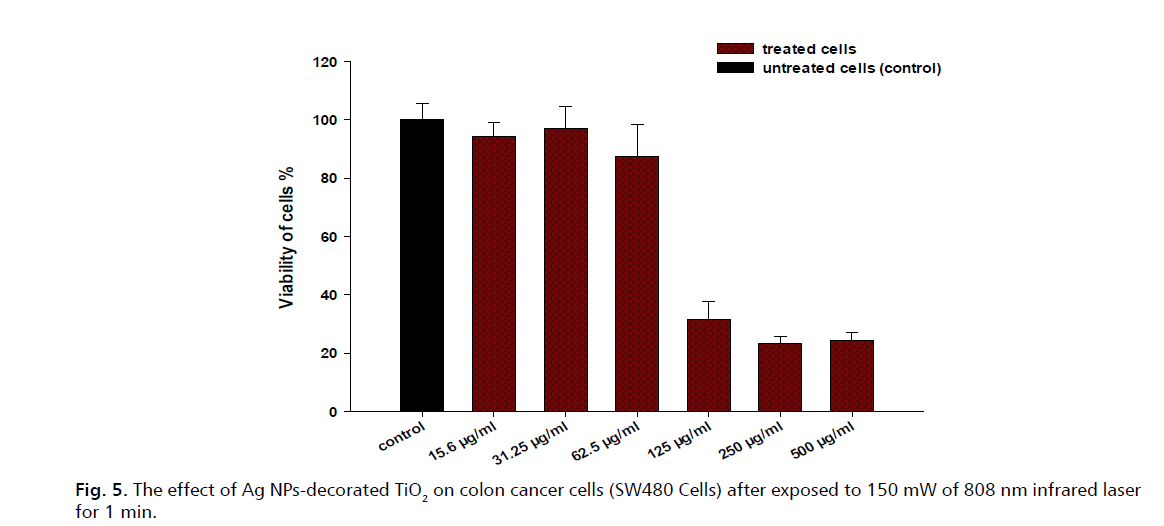
Figure 5: The effect of Ag NPs-decorated TiO2 on colon cancer cells (SW480 Cells) after exposed to 150 mW of 808 nm infrared laser for 1 min.
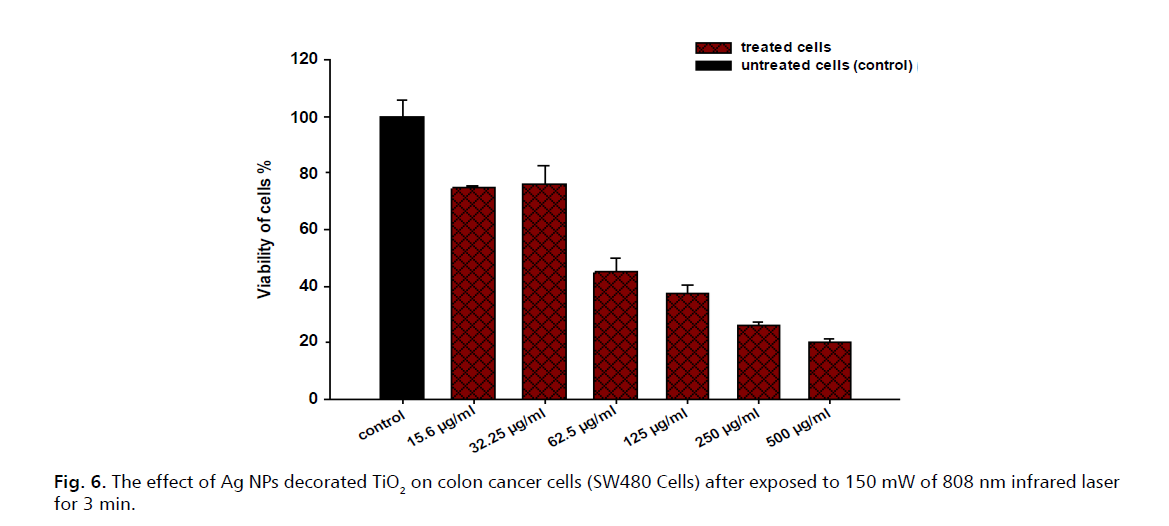
Figure 6: The effect of Ag NPs decorated TiO2 on colon cancer cells (SW480 Cells) after exposed to 150 mW of 808 nm infrared laser for 3 min.
From Fig. 6., it can be inferred that the decrease in cell viability with increasing concentrations of Ag NPs-decorated TiO2 could be due to the induction of apoptosis in the SW480 cells. The 808 nm Infrared laser has been used in photobiomodulation therapy, which has been shown to regulate cell differentiation [28]. In the case of the experiment, the laser could have enhanced the effect of the Ag NPs-decorated TiO2 on the SW480 cells, leading to the observed decrease in cell viability.
The effect of Ag NPs decorated TiO2 on colon cancer cells (SW480 Cells) after Exposed to 150 mW of 532 nm green laser for 3 min is shown in Fig. 7. The cells were treated with different concentrations of the nanoparticles and then irradiated with 300 mW of 532 nm laser for 3 minutes. The cell viability was measured by MTT assay and expressed as a percentage of the control group (untreated cells).
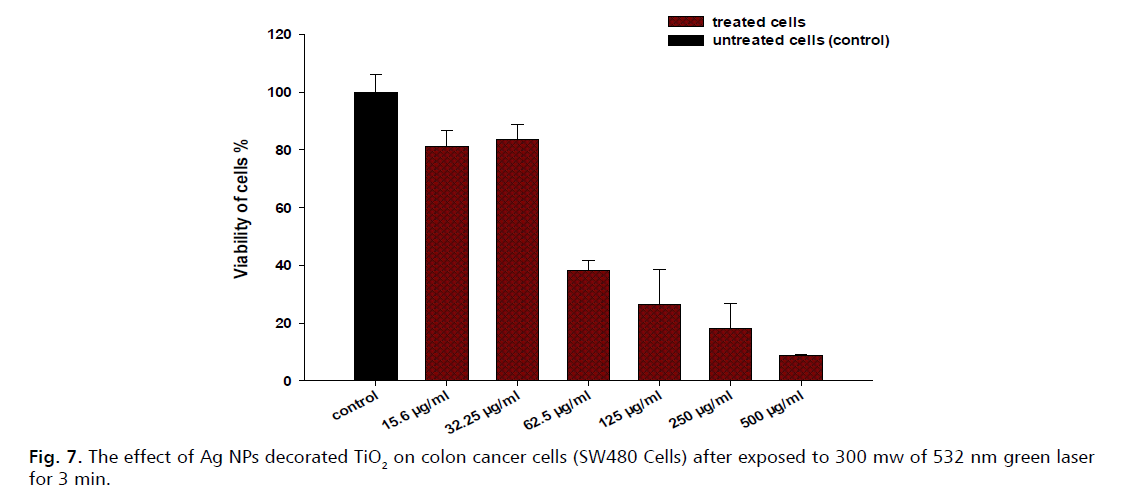
Figure 7: The effect of Ag NPs decorated TiO2 on colon cancer cells (SW480 Cells) after exposed to 300 mw of 532 nm green laser for 3 min.
The results indicate that the treatment with Ag NPs-decorated TiO2 and green laser significantly reduced the cancer cell viability in a dose-dependent manner. The control group showed near 100% viability, while the lowest concentration (15.6 µg/mL) resulted in about 80% viability. As the concentration increased, the viability decreased sharply, reaching less than 10% at both 250 and 500 µg/mL. This suggests that the nanoparticles enhanced the photothermal effect of the laser, causing more damage to the cancer cells.
The mechanism of this effect can be explained by the interaction between the nanoparticles and the laser light. Ag NPs-decorated TiO2 have high absorption and scattering properties in the visible and near-infrared regions, which enable them to convert light energy into heat. When the nanoparticles are internalized by the cells, they generate localized heating that can induce cell death by various pathways, such as apoptosis, necrosis, or autophagy. The higher the concentration of the nanoparticles, the more heat is generated and the more cancer cells are killed.
Cell viability decreases with increasing nanoparticle concentration, the results indicate that the treatment with Ag NPs-decorated TiO2 and green laser significantly reduces the viability of SW480 cells in a dose-dependent manner. At the lowest concentration (15 µg/mL), the cell viability is about 90%, while at the highest concentration (500 µg/mL), it drops to about 10%. The control group shows almost 100% cell viability, indicating that the laser alone does not affect the cells. Nanoparticles enhance the photothermal therapy of cancer cells, the mechanism behind this effect is likely the photothermal conversion of the nanoparticles, which absorb the green laser light and generate heat. This heat damages the cancer cells and induces apoptosis (programmed cell death). The higher the concentration of nanoparticles, the more heat is generated and the more cells are killed.
Nanoparticles have potential for cancer treatment, this study demonstrates the potential of Ag NPs-decorated TiO2 as a novel nanomaterial for photothermal therapy of colon cancer. This approach has several advantages over conventional therapies, including minimal invasiveness, and reduced side effects. However, further research is needed to optimize the parameters of the treatment, such as nanoparticle size, shape, and surface modification, as well as laser intensity, wavelength, and duration. Moreover, the biocompatibility, biodistribution, and toxicity of the nanoparticles should be evaluated in vivo before clinical application. This study is consistent with the latest scientific sources on the use of nanoparticles for photothermal therapy of cancer. Qamandar, et al. [29] in 2024 summarized the advances and challenges of this approach, highlighting the advantages of Ag NPs and TiO2 as photothermal agents. Another study suggests that Ag NPs-decorated TiO2 have great potential for cancer treatment by photothermal therapy [30].
CONCLUSION
The purpose of this study is to investigate the toxicity of Titanium dioxide (TiO2) nanotubes arrays decorated with silver nanostructures and to evaluate their photothermal anticancer effect in Colon cancer cells by laser irradiation. Several analyses were applied to support the findings. TEM micrographs of the samples confirmed that the TiO2 nanotubes are decorated by Ag nanoparticles. Moreover, XRD pattern of Ag NPs decorated TiO2 nanotubes inferred the successful decoration of Ag on the TiO2 nanostructures which is in agreement with the EDX data. A cellular toxicity examination was carried out using laser therapy on SW480 colon cancer cell lines to study the poisonous effects of Ag NPs decorated TiO2 nanotubes. This investigation aims to find out various doses as well as efficient concentrations of Ag@TiO2 used for low-energy laser treatment. The higher concentration (125,250 and 500µg/ml) of Ag NPs decorated TiO2 had a better anticancer effect on SW480 cell line. The combination results of green (532nm) and IR (808nm) laser at exposure time 3 minute had a better anticancer effect after 24 hours specifically at (62.5, 125, 250 and 500µg/ml) of Ag NPs decorated TiO2. The green and infrared lasers had a synergism effect by reducing the cell number, the effect was dose-time dependent.
FUNDING
This research did not receive any specific grant from funding agencies.
DECLARATIONS
The authors declare that they have no conflict of interest.
REFERENCES
- Lee JY. Book Review: Cancer as a metabolic disease: On the origin, management, and prevention of cancer. J Gynecol Oncol. 2014; 25(3):260-261.
- Chanu MT, Singh AS. Different types of cancer treatment, its advancement, benefits, and side effects.
- Youkhana EQ, Feltis B, Blencowe A, Geso M. Titanium dioxide nanoparticles as radiosensitisers: An in vitro and phantom-based study. Int J Med Sci. 2017; 14(6):602.
- Dixit AA, Mandlik DS, Mandlik SK. Functionalised ligand-based nanomaterial drug targeting approaches for colorectal cancer therapy. Recent Advances in Drug Delivery and Formulation: Formerly Recent Patents on Drug Delivery & Formulation. 2024; 18(3):170-187.
- Narayana S, Gowda BJ, Hani U, Shimu SS, Paul K, et al. Inorganic nanoparticle-based treatment approaches for colorectal cancer: recent advancements and challenges. J Nanobiotechnol. 2024; 22(1):427.
- Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, et al. Recent advances in the treatment of colorectal cancer: a review. J Nippon Med School. 2022; 89(3):246-254.
- Rahimi G, Yousefnia S, Angnes L, Negahdary M. Design a PEGylated nanocarrier containing Lemongrass Essential Oil (LEO), a drug delivery system: Application as a cytotoxic agent against breast cancer cells. J Drug Deliv Sci Tech. 2023; 80:104183.
- Nasri N, Saharkhiz S, Dini G, Yousefnia S. Thermo-and pH-responsive targeted lipid-coated mesoporous nano silica platform for dual delivery of paclitaxel and gemcitabine to overcome HER2-positive breast cancer. Int J Pharma. 2023; 648:123606.
- Saharkhiz S, Nasri N, Dini G, Yousefnia S. Development of a new smart theranostic anti-PSMA-aptamer conjugated cationic-lipid coated mesoporous silica platform for targeted delivery of paclitaxel and CdSe/ZnS quantum dots to LNCaP cell line. J Drug Deliv Sci Tech. 2023; 88:104964.
- Zarzzeka C, Goldoni J, Marafon F, Sganzerla WG, Forster-Carneiro T, et al. Use of titanium dioxide nanoparticles for cancertreatment: A comprehensive review and bibliometric analysis. Biocatal Agric Biotechnol. 2023; 50:102710.
- Sharma M, Thakur P, Gaur P, Rani GM, Rustagi S, et al. Cancer treatment and toxicity outlook of nanoparticles. Environ Res. 2023; 237:116870.
- Hsu CY, Mahmoud ZH, Abdullaev S, Ali FK, Naeem YA, et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud Chem Environ Eng. 2024; 9:100626.
- Gholizadeh Z, Aliannezhadi M, Ghominejad M, Tehrani FS. Novel boehmite and η-alumina nanostructures synthesized using a green ultrasonic-assisted hydrothermal method by clove extract for water treatment. J Water Process Eng. 2024; 65:105786.
- Cui H, Zhu W, Yue C, Yang M, Ren W, et al. Recent progress of ultrasound-responsive titanium dioxide sonosensitizers in cancer treatment. Chin Chem Lett. 2024:109727.
- Al-Shemri MI, Aliannezhadi M, Ghaleb RA, Al-Awady MJ. Au-H2Ti3O7 nanotubes for non-invasive anticancer treatment by simultaneous photothermal and photodynamic therapy. Sci Rep. 2024; 14(1):25998.
- Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007; 59(8):748-758.
- Seçme A, Bozer BM, Kocaman AY, Erenler R, Calimli MH. Synthesis, characterization, and anticancer properties of Ag nanoparticles derived from walnut leaves tested on cells of L929, MCF-7 and H1299. J Drug Deliv Sci Tech. 2024; 94:105478.
- Kim D, Yu MK, Lee TS, Park JJ, Jeong YY, et al. Amphiphilic polymer-coated hybrid nanoparticles as CT/MRI dual contrast agents. Nanotechnol. 2011; 22(15):155101.
- Gholizadeh Z, Aliannezhadi M, Ghominejad M, Shariatmadar Tehrani F. Optical and structural properties of spherical-shaped boehmite and γ-alumina nanoparticles by ultrasonic-assisted hydrothermal method: the effects of synthesis route, calcination, and precursor concentration. Opt Quantum Electron. 2023; 55(10):880.
- Fakhredin M, Tehrani FS, Aliannezhadi M. The role of reaction temperature in synthesizing clove-derived copper oxide nanoparticles for brain cancer treatment. Results in Physics. 2025; 68:108101.
- Al-Shemri MI, Aliannezhadi M, Al-Awady MJ, Ghaleb RA. Interaction of different lasers beams with synthesized H2Ti3O7 nanotubes: toward photodynamic therapy. Opt Quantum Electron. 2023; 55(8):671.
- Narasimha VR, Latha TS, Pallu R, Panati K, Narala VR. Anticancer activities of biogenic silver nanoparticles targeting apoptosis and inflammatory pathways in colon cancer cells. J Clust Sci. 2022; 33(5):2215-31.
- Bao J, Jiang Z, Ding W, Cao Y, Yang L, et al. Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells. Nanotechnol Rev. 2022; 11(1):1911-26.
- Akter M, Atique Ullah AK, Banik S, Sikder MT, Hosokawa T, et al. Green synthesized silver nanoparticles-mediated cytotoxic effect in colorectal cancer cells: NF-κB signal induced apoptosis through autophagy. Biol Trace Elem Res. 2021; 199:3272-3286.
- Althomali A, Daghestani MH, Basil Almukaynizi F, Al-Zahrani SA, Awad MA, et al. Anti-colon cancer activities of green-synthesized Moringa oleifera–AgNPs against human colon cancer cells. Green Process Synth. 2022; 11(1):545-554.
- Dheaa DJ, Mahdi SA. Photodynamic Therapy and TiO2-Decorated Ag Nanoparticles: Implications for Skin Cancer Treatment. J Composite & Adv Mater /Revue des Composites et des Matériaux Avancés. 2023; 33(6).
- Wang X, Wang Z, Jiang X, Tao J, Gong Z, et al. Silver-decorated TiO2 nanorod array films with enhanced photoelectrochemical and photocatalytic properties. J Electrochem Soc. 2016; 163(10):H943.
- Amaroli A, Sabbieti MG, Marchetti L, Zekiy AO, Utyuzh AS, et al. The effects of 808-nm near-infrared laser light irradiation on actin cytoskeleton reorganization in bone marrow mesenchymal stem cells. Cell and tissue research. 2021; 383:1003-1016.
- M. A. Qamandar. “Exploring the Biological Applications of TiO2 and Ag NPs: Innovations and Impacts.” International Journal of Medical Science and Dental Health.2024; 10(8): 12-26.
- Nie C, Du P, Zhao H, Xie H, Li Y, et al. Ag@ TiO2 nanoprisms with highly efficient near‐infrared photothermal conversion for melanoma therapy. Chemistry–An Asian Journal. 2020; 15(1):148-155.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at



