Review Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 6
Energy metabolism therapy for Brain Cancer
Tejasvi* and Amit NayakTejasvi, Department of School of Medical and Allied Sciences, GD Goenka University, Haryana, India, Email: choudharytejaswi@gmail.com
Received: 12-Apr-2023, Manuscript No. OAR-23-96297; Accepted: 30-May-2023, Pre QC No. OAR-23-96297 (PQ); Editor assigned: 16-Apr-2023, Pre QC No. OAR-23-96297 (PQ); Reviewed: 02-May-2023, QC No. OAR-23-96297 (Q); Revised: 27-May-2023, Manuscript No. OAR-23-96297 (R); Published: 02-Jun-2023
Abstract
Brain tumours that are malignant are a serious health issue that can affect both children and adults and are frequently difficult to treat. In any case, late examination recommends that adjustments of metabolic climate could be a promising arrangement. It may be possible to manage brain cancer more effectively by combining metabolic control analysis with the capacity of normal cells to survive extreme changes in the physiological environment. By switching the bioenergetics source from glucose to ketone bodies, this strategy targets brain tumours through integrated anti-inflammatory, anti-angiogenic, and pro-apoptotic mechanisms. Both orthotropic mouse brain tumour models and human paediatric astrocytomas treated with dietary energy restriction and the ketogenic diet have successfully tested this strategy.
Keywords
cancer pain, pain assessment, pain management, cross-sectional study, literature review
Introduction
Malignant brain tumours may be on the rise worldwide, affecting both children and the elderly. However, despite this concerning trend, the standard treatments for these tumours, including surgical resection and radiation, have remained largely unchanged for over fifty years. While these treatments may initially slow tumour growth, they can ultimately facilitate recurrence and increase growth rates through changes in morphogenetic fields. Chemotherapy is also of limited benefit for most malignant gliomas and can cause negative side effects that reduce quality of life. Therapeutic targeting of brain tumour associated mutations is complicated by the fact that these mutations may arise as by-products of tissue disorganization, and their causal relationship is unclear. Although some progress has been made with temozolomide chemotherapy, it is clear that new approaches are needed to manage malignant brain tumours effectively and maintain a decent quality of life for patients [1-10].
Literature Review
Analyses of metabolic control
Metabolic control analysis is a method of analyzing and treating complex diseases by evaluating the degree of flux in metabolic pathways. This approach is based on the fact that compensatory genetic and biochemical pathways regulate the bioenergetics potential of cells and ultimately the phenotype. Abnormal energy metabolism and biological chaos are characteristics of brain tumours, and the general principles of metabolic control analysis can be effective for brain cancer management. The hypothesis is based on the known differences in energy metabolism between normal and neoplastic brain cells. New therapeutic approaches, which lower circulating glucose and elevate ketone bodies, target brain tumours while enhancing the metabolic efficiency of normal neurons and glia. This approach involves global manipulations of metabolic networks to restore orderly adaptive behaviour to widely disordered states involving complex gene-environmental interactions [11-20].
Metabolism of energy in normal brain cells
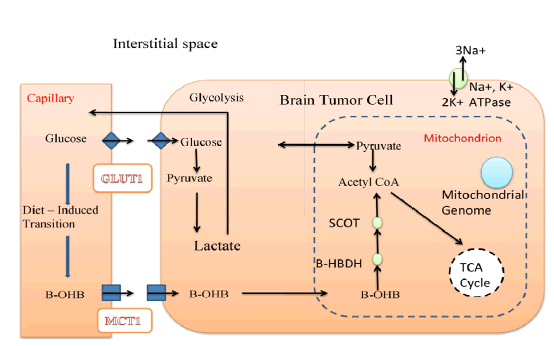
The management of brain cancer through metabolic targeting involves taking into consideration the energy metabolism of the normal orthotropic tissue (Figure 1). A detailed understanding of the metabolic pathways involved is essential for effective management. In normal physiological conditions, the mature brain primarily derives its energy from the aerobic oxidation of glucose, with almost all of the glucose metabolized to pyruvate, which is converted to Acetyl CoA before entering the TCA cycle.
Figure 1:A detailed understanding of the metabolic pathways involved is essential for effective management.
Lactate is not used for energy metabolism in the adult brain, although there is some controversy surrounding this claim. Fatty acids do not pass through the blood-brain barrier as fuel substrates, except for octanoate, which may be an exception. Therefore, glucose is the primary, if not exclusive, metabolic fuel for the brain under normal physiological conditions [21-25].
However, under fasting-induced reductions of blood glucose, neurons and glia will metabolize ketones for energy. This is a physiological adaptation to prolonged food restriction and is conserved across species. Ketone bodies, consisting of acetoacetate and β-hydroxybutyrate (β-OHB), are derived from fat catabolism in the liver, and their concentration in the blood is inversely related to that of glucose. Ketone bodies are transported into the brain through the blood-brain barrier monocarboxylic transporters (MCTs), whose expression is regulated, in part, by circulating ketone and glucose levels [15,26-29].
β-OHB is the predominant blood ketone body and is rapidly oxidized to Acetyl CoA in the mitochondria through an enzymatic series involving 3-hydroxybutyrate dehydrogenase, SCOT (succinyl-CoA-acetoacetate-CoA transferase), and mitochondrial acetyl-CoA thiolase. Acetone is a non-enzymatic by product of ketone body synthesis and is largely excreted in the urine or exhaled from the lungs. Although the levels of glucose and ketones in the brain are proportional to their levels in the blood, the adult brain does not usually metabolize ketone bodies for energy unless blood glucose levels are reduced [30-34].
The therapeutic efficacy of the ketogenic diet is best achieved when coupled to dietary energy restriction, under which conditions circulating glucose levels are gradually reduced, and ketone body levels are elevated. It is essential to emphasize the term "gradual" as ketone bodies cannot be used for energy following acute hypoglycaemia. However, in vitro preparations show that neuronal glial interactions are disrupted, and the blood-brain barrier is absent. Therefore, the gradual transition from glucose to ketone bodies for energy in vivo requires a flexible genome for the coordinated integration of multiple metabolic networks according to principles of metabolic control analysis [35, 36].
Ketone bodies are more energetically efficient than either pyruvate or fatty acids because they are more reduced, with a greater hydrogen/carbon ratio than pyruvate, and do not uncouple the mitochondrial proton gradient as occurs with fatty acid metabolism. Ketone bodies bypass cytoplasmic glycolysis and directly enter the mitochondria, where they are oxidized to Acetyl CoA. The amount of Acetyl-CoA formed from ketone body metabolism is also greater than that formed from glucose metabolism, which increases TCA cycle metabolites while reducing the mitochondrial NAD couple, (NAD+)/(NADH), and increasing the mitochondrial Q couple (Q)/(QH2) [14,16]. The difference between these couples enhances the mitochondrial proton gradient, which, in turn, enhances the energy available from the hydrolysis of ATP, ∆G'ATP, the cell's key energy reserve generated through the mitochondrial Fl ATPase. Remarkably, the ketone body-induced increase in the ∆G'ATP is also accomplished using less oxygen. These and other findings led Vetch to designate ketone bodies as a super fuel.
Free radicals and ketone bodies
Ketone body digestion offers not just the benefit of expanding ATP creation while diminishing oxygen utilization, yet it additionally can bring down the development of destructive free revolutionaries. The semi Quinone of Q, which is the partially reduced form of coenzyme Q, is found to spontaneously react with oxygen and be the primary source of mitochondrial free radical generation. Notwithstanding, the oxidation of the several reductions how much the semi Quinone structure, in this way lessening superoxide creation. The reduced form of glutathione, which is nearly in equilibrium with the cytosolic free NADP+/ NADPH concentration couple, is also increased by ketone body metabolism, making hydrogen peroxide elimination easier. Ketone metabolism reduces tissue inflammation caused by reactive oxygen species by reducing free radicals. In conclusion, ketone bodies possess anti-inflammatory properties in addition to being a superior metabolic fuel to glucose [37-40].
Energy utilization in tumours of the brain
The normal brain can use both glucose and ketone bodies as sources of energy. However, malignant brain tumours, whether in humans or animal models, are not able to switch between these energy sources and rely mostly on glucose for energy. This is due to enhanced glycolysis that produces excess lactic acid, which can be recycled back to the tumour as glucose. Ketone body metabolism in tumours is mainly used for lipid synthesis rather than for energy production [41, 42].
Brain tumours have reduced activity of the rate-controlling step for utilizing β-OHB as a respiratory fuel, which prevents them from using ketone bodies as an alternative energy source. Additionally, most tumours, including brain tumours, have abnormalities in the number and function of their mitochondria, which are required for the oxidation of ketone bodies [43, 44].
Brain tumours exhibit increased glycolysis and lactic acid production, coupled with reduced respiratory capacity. The abnormality in respiratory capacity involves alterations in the TCA cycle components, electron transport, and deficiencies in oxidative phosphorylation. These defects make brain tumour cells vulnerable to metabolic targeting through metabolic control analysis [45, 46].
The Warburg hypothesis suggested that the increased glycolysis of tumour cells occurs gradually to compensate for respiratory failure. In contrast to normal brain cells, where glycolysis and respiration are tightly coupled, tumour cells are defective in integrating energy metabolism between glycolysis and respiration. Activation of the oncogene may render cancer cells dependent on aerobic glycolysis for continued growth and survival. Recent studies suggest that disconnect between respiration and glycolysis renders cancer cells vulnerable to metabolic targeting through metabolic control analysis. Studies with the ketogenic diet and dietary energy restriction support this possibility. [40-46]
Dietary energy and cerebrum disease
The ketogenic diet
In 1995, a pioneering study was conducted by Nebeling and colleagues, which explored the use of a high-fat, low-carbohydrate diet known as the Ketogenic Diet (KD) for treating malignant brain cancer in humans. This diet had already been used for many years as a successful therapy for refractory seizures in children [15, 48, and 49]. The primary objective of the study was to alter the primary substrate for energy metabolism from glucose to ketone bodies, in order to disrupt tumour metabolism while simultaneously maintaining the nutritional status of patients [47].
The study included two female children with non-resectable advanced grade brain tumours, anaplastic astrocytoma stage IV and cerebellar astrocytoma stage III. Despite extensive radiation and chemotherapy, measurable tumour remained in both subjects. However, both children responded exceptionally well to the KD and experienced long-term tumour management without further chemo or radiation therapy [47]. In fact, as of the time of writing, one of the patients was still alive and well (Nebeling, personal communication). Positron Emission Tomography with fluro-deoxyglucose (FDG-PET) also demonstrated a 21.8% reduction in glucose uptake at the tumour site in both subjects on the KD [47].
Despite the sound logic and significant findings of this study, no further human studies or clinical trials have been conducted on the therapeutic efficacy of the KD for brain cancer. The reasons for this are unclear, but it may be due to the preference of the major Brain Tumour Consortia for using "hand-me-down" drug therapies from other cancer studies, rather than exploring more effective biological or non-chemotherapeutic approaches [7]. This is unfortunate, as recent findings in animal models of brain tumours suggest that the therapeutic potential of the restricted KD, which involves reduced glucose and elevated β-OHB, is likely greater than that of any current brain tumour chemotherapy [20]. Furthermore, the KD would eliminate or reduce the need for adjuvant anticonvulsant and steroidal medications for brain tumour patients, as the KD has antiepileptic and anticonvulsant effects and, when restricted in caloric intake, naturally elevates circulating glucocorticoid levels [15, 49-51].
These findings suggest that the KD could be an effective multifactorial diet therapy for malignant brain cancer and should be considered as an alternative therapeutic option.
Dietary energy limitation:
Recently, the findings of the Nebeling group on the efficacy of the Ketogenic Diet (KD) for treating brain cancer were confirmed in a series of orthotropic mouse brain tumour models that were treated with the KD and dietary energy restriction [37,32, 52, 53]. The KD is a high-fat, low-carbohydrate diet that has been used for decades to treat refractory seizures in children, and the objective of the Nebeling study was to disrupt tumour metabolism by shifting the primary substrate for energy metabolism from glucose to ketone bodies while maintaining the nutritional status of patients.
The DR-induced inhibition of brain tumour growth is directly correlated with reduced levels of glucose and elevated levels of ketone bodies [20, 37]. DR is a natural dietary therapy that improves health, prevents tumour formation, and reduces inflammation . DR also improves mitochondrial respiratory function and glutathione redox state in normal cells. Thus, DR naturally inhibits glycolysis and tumour growth while enhancing the health and vitality of normal cells and tissues.
The anti-angiogenic effects of DR arise from reduced tumour energy metabolism [37, 32, 52, 53]. The antigenic properties of most human gliomas are closely linked to metabolic activity, and DR reduces cerebral blood flow and oxygen consumption, which further stress brain tumour cells that are already weakened from reduced glucose levels [28]. In addition to reducing angiogenesis, DR also increases brain tumour apoptosis [52, 53]. The proapoptotic effects of DR occur in large part from the reduction in glycolytic energy that most tumours rely on for growth. This reduction in glycolytic energy would reduce lactate levels and hydroxyl radical production, which are known to enhance tumour inflammation and cytokine production through glial activation [54-57].
DR also reduces inflammation and the inflammatory properties of macrophages while enhancing their phagocytic activities. An uncoupling of the detrimental inflammatory properties of tumour associated macrophages from their beneficial phagocytic properties is considered essential for the eventual management of brain cancer. Hence, diet therapies that lower glucose availability and elevate ketone bodies can reduce brain tumour growth through integrated anti-inflammatory, anti-angiogenic, and proapoptotic mechanisms.
It is worth noting that previous studies have shown that the antitumour effects of DR result from caloric restriction per se and not from the restriction of any specific dietary component, such as proteins, vitamins, minerals, fats, or carbohydrates [36, 54, 55]. Despite the logic of these studies and the dramatic findings, no further human studies or clinical trials have been conducted on the therapeutic efficacy of the KD for brain cancer. The reason for this is not clear but may reflect a preference of the major Brain Tumour Consortia for using "hand-me-down" drug therapies from other cancer studies rather than exploring more effective biological or non-chemotherapeutic approaches [7]. However, recent findings in brain tumour animal models suggest that the therapeutic potential of the restricted KD, involving reduced glucose and elevated β-OHB, is likely to be greater than that of any current brain tumour chemotherapy [20]. Moreover, the KD would eliminate or reduce the need for adjuvant anticonvulsant and steroidal medications for brain tumour patients, as the KD has antiepileptic and anticonvulsant effects, and when restricted in caloric intake, it will naturally elevate circulating glucocorticoid levels [15, 49-51]. These findings suggest that the KD would be an effective multifactorial diet therapy for malignant brain cancer and should be considered as an alternative therapeutic option.
Brain Cancer's Metabolic Control: A Developmental Viewpoint:
The authors of this article suggest a new approach to managingbrain cancer that focuses on the differences in energy metabolism between normal brain cells and tumour cells. They propose combining metabolic control analysis with the evolutionary capacity of normal cells to adapt to extreme shifts in physiological environment to create a novel strategy for brain cancer management [58, 26].
According to the authors, the adaptation of normal cells to environmental extremes is conserved within the genome, and only cells with a flexible genome will be capable of surviving abrupt changes in metabolic landscape. Cells with genomic defects that limit flexibility should be less adaptable to metabolic stress and therefore vulnerable to elimination through principles of metabolic control analysis. This approach focuses more on the genetic capabilities of normal cells than on the genetic defects of tumour cells [59, 60].
Brain cancer is a metabolic disorder that involves the dysregulation of glycolysis and respiration, which can be managed through abrupt changes in metabolic environment. The amount of energy needed to maintain the activity of transmembrane ion pumps is greater than that needed for mitosis, and the survival of cells depends on maintaining an adequate ∆G' of ATP hydrolysis. Tumour cells with limited genomic flexibility should therefore be less capable than normal cells in utilizing alternative energy substrates to maintain their ∆G' of ATP hydrolysis [16, 61, 52, 62].
The energy used to maintain pump function and cell viability in normal brain cells comes from either glycolysis or aerobic respiration. In the case of brain tumours, this energy is mostly derived from glycolysis, making them vulnerable to reductions in circulating glucose levels as these mutant cells would have difficulty oxidizing alternative fuels (ketone bodies) through respiration [63, 47].
The authors propose a strategy for managing brain tumours that enhances the respiratory potential of normal brain cells while metabolically targeting tumour cells. The approach would involve a sequential series of therapeutic steps and should be effective against any primary or secondary brain tumour regardless of cell of origin, anatomical location, or histological grade. Step one would lower circulating glucose levels and elevate circulating β-OHB levels through diet therapies or ketone body supplementation. Glucose ranges between 3.0-3.5 mm (55–65 mg/dl) and β-OHB ranges between 4-5 mm should be effective for tumour management in most patients [29, 30, 65-66].
The authors suggest that reduced glucose and elevated ketones could also antagonize tumour cachexia. The proposed strategy could be used to indirectly target genetically defective and less metabolically flexible brain tumour cells by exploiting the genomic and metabolic flexibility of normal brain cells. The authors conclude that this new approach could lead to the development of effective clinical therapies for brain tumours [58].
Step two of this proposed therapeutic strategy involves the surgical removal of brain tumours if necessary. Smaller tumours with clear boundaries and reduced blood flow are easier to remove than larger tumours with poorly defined boundaries and extensive blood flow. After surgery, the diet therapy can be adjusted to aid in healing and maintain metabolic pressure on any remaining tumour cells. Step three involves the use of conventional or novel targeted therapies, which may not have long-term benefits initially, but can be highly effective after weakening and metabolically isolating the tumour cells through the first two steps. Glycolysis inhibitors, which may harm normal cells, could also be more effective at this stage. Weight cycling strategies and specific amino acid restrictions may also aid in eradicating or slowing tumour cell growth. The goal of this approach is to consistently alter the physiological and metabolic environment of both the tumour and host, with only the most adaptable cells expected to survive. This approach is based on principles of evolutionary biology and metabolic control analysis, and is expected to be more successful than current approaches [59-70].
References
- No meeting of minds on childhood cancer
- Lowry JK, Snyder JJ, Lowry PW. Brain tumours in the elderly: recent trends in a Minnesota cohort study. J Neurol. 1998; 7:922-928.
- Kaatsch P, Rickert CH, Kühl J, Schuz J, Michaelis J, et al. Populationâ?based epidemiologic data on brain tumours in German children. CA Cancer J Clin. 2001; 92: 3155-3164.
- Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG et al. Trends in incidence of primary brain tumours in the United States, 1985-1994. Neuro-Oncol. 2001; 3:141-151.
- Zimmerman HM. The nature of gliomas as revealed by animal experimentation. Am J Clin Pathol. 1955; 31: 1.
- Chang SM, Parney IF, Huang W, Anderson FA, Asher AL, et al. Patterns of care for adults with newly diagnosed malignant glioma. Jama. 2005; 293: 557-564.
- Fisher PG, Buffler PA. Malignant gliomas in 2005: where to GO from here?. Jama. 2005; 293: 615-617.
- Seyfried, T. N. Perspectives on brain tumor formation involving macrophages, glia, and neural stem. Perspect Biol Med, 44, 263-282.
- Sonnenschein C, Soto AM. The Society of Cells: Cancer Control and Proliferation. 1999
- Shapiro WR. Current therapy for brain tumors: back to the future. J Neurol. 1999; 56:429-432.
- Sonnenschein C, Soto AM. Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. Mol Cancer Ther. 2000; 29:205-211.
- Kiebish MA, Seyfried TN. Absence of pathogenic mitochondrial DNA mutations in mouse brain tumors. BMC cancer. 2005; 5:1-8.
- Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;10: 352:987-996.
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fat Acids. 2004; 70: 309-319.
- Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J. Neurochem. 2003; 86:529-537.
- Veech RL. Metabolic control analysis of ketone and insulin action: Implications for phenotyping of disease and design of therapy. 2002; 21.
[Google Scholar] [CrossRef]
- Strohman R. Maneuvering in the complex path from genotype to phenotype. Science. 2002; 296:701-703.
- Kacser H, Burns JA. The molecular basis of dominance. Genetics. 1981; 97:639-666.
- Greenspan RJ. The flexible genome. Nat Rev Genet. 2001; 2:383-387.
- Seyfried TN, Mukherjee P. Anti-angiogenic and pro-apoptotic effects of dietary restriction in experimental brain cancer: role of glucose and ketone bodies. Integration/interaction of oncologic growth. 2005:259-270.
- Lowry, O. H., Passonneau, J. V., Hasselberger, F. X., Schulz, D. W. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem. 1964; 239: 18-30.
- Djavani, M., Crasta, O. R., Zhang, Y., Zapata, J. C., Sobral, B., Lechner, M. G., & Salvato, M. S. (2009). Gene expression in primate liver during viral hemorrhagic fever. Virol J. 2009; 6: 1-18.
- Allen NJ, Káradóttir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005; 25:848-859.
- Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U.S.A. 1998; 95: 3990-3995.
- Pellerin L, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005; 79: 55-64.
- Cahill Jr, G. F. Cahill Jr GF. Starvation in man. N Engl J Med. 1970; 282: 668-675.
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, et al. Brain metabolism during fasting. J Clin Invest. 1967; 46:1589-1595.
- VanItallie TB, Nufert TH. Ketones: metabolism's ugly duckling. Nutr Res Rev. 2003; 61: 327-341.
- Morris AA. Cerebral ketone body metabolism. J Inherit Metab. 2005; 28: 109-121.
- Veech, R. L. Cahill Jr GF, Veech RL. Ketoacids? good medicine?. Trans Am Clin Climatol Assoc. 2003; 114: 149-161.
- Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN et al. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab. 2004; 1: 1-1.
- Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004; 10: 5622-5629.
- Fredericks M, Ramsey RB. 3â?Oxo acid coenzyme A transferase activity in brain and tumours of the nervous system 1. J Neurochem 1978; 31: 1529-1531.
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill Jr GF et al. Ketone bodies, potential therapeutic uses. IUBMB life. 2001; 51: 241-247.
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, et al. d-β-Hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A. 2000; 97: 5440-5444.
- Masuda R, Monahan JW, Kashiwaya Y. Dâ?βâ?hydroxybutyrate is neuroprotective against hypoxia in serumâ?free hippocampal primary cultures. Proc Natl Acad Sci U S A. 2005; 80: 501-509.
- Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P, et al. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003; 89: 1375-1382.
[Google Scholar] [CrossRef]
- Mangiardi JR, Yodice P. Metabolism of the malignant astrocytoma. Neuro. 1990; 26: 1-9.
- Rhodes CG, Wise RJ, Gibbs JM, Frackowiak RS, Hatazawa J, et al. In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Proc Natl Acad Sci U.S.A. 1983; 14: 614-626.
- Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, et al. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer research. 1997; 17: 1903-1911.
- Patel MS, Russell JJ, Gershman, H. Ketone-body metabolism in glioma and neuroblastoma cells. Proc Natl Acad Sci U S A. 1981; 78: 7214-7218.
- Roeder LM, Poduslo SE, Tildon JT. Utilization of ketone bodies and glucose by established neural cell lines. J neuro res. 1982; 8: 671-682.
- Aisenberg, A. C. The glycolysis and respiration of tumors. Academic press. 1961.
- Ikezaki K, Black KL, Conklin SG, Becker DP. Histochemical evaluation of energy metabolism in rat glioma. Neuro Res. 1992; 14: 289-293.
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000; 39: 257-288.
- Canuto RA, Biocca ME, Muzio G, Dianzani MU. Fatty acid composition of phospholipids in mitochondria and microsomes during diethylnitrosamine carcinogenesis in rat liver. Mol Cell Biochem. 1989; 7: 11-19.
- Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14: 202-208.
- Stafstrom CE, Bough KJ. The ketogenic diet for the treatment of epilepsy: a challenge for nutritional neuroscientists. Nutr Neurosci. 2003; 6: 67-79.
- Freeman JM, Freeman JB, Kelly MT. The ketogenic diet: a treatment for epilepsy. Demos Medical Publishing; 2000.
[Google Scholar] [CrossRef]
- Zhu Z, Jiang W, Thompson HJ. Mechanisms by which energy restriction inhibits rat mammary carcinogenesis: in vivo effects of corticosterone on cell cycle machinery in mammary carcinomas. Carcinog. 2003; 24: 1225-1231.
- Patel NV, Finch CE. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol Aging. 2002; 23: 707-717.
- Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res. 2004; 10: 5622-5629.
- Mukherjee P, El-Abbadi MM, Kasperzyk JL, Ranes MK, Seyfried TN, et al. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer. 2002; 86: 1615-1621.
- Tannenbaum A. The genesis and growth of tumors. II. Effects of caloric restriction per se. Cancer Res. 1942; 2: 460-467.
- Mukherjee P, Sotnikov AV, Mangian HJ, Zhou JR, Visek WJ, et al. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst. 1999; 91: 512-523.
- Pennathur S, Ido Y, Heller JI, Byun J, Danda R, et al. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005; 280: 22706-22714.
- Andersson AK, Rönnback L, Hansson E. Lactate induces tumour necrosis factorâ?α, interleukinâ?6 and interleukinâ?1β release in microglialâ?and astroglialâ?enriched primary cultures. J neuro. 2005; 93: 1327-1333.
- Potts R. Humanity’s Descent: The Consequences of Ecological Instability. William Morrow & Co.
- Harold FM. The Vital Force: A Study of Bioenergetics WH Freeman and Company. New York. 1986.
- Google Scholar
- Lipton P, Robacker K. Glycolysis and brain function:[K+] o stimulation of protein synthesis and K+ uptake require glycolysis. InFederation proceedings. 1983; 42: 2875-2880.
- Strohman R. Thermodynamics-old laws in medicine and complex disease. Nat Biotechnol. 2003; 21: 477-479.
- Nebeling LC, Lerner E. Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. Proc Natl Acad Sci U S A. 1995; 95: 693-697.
- Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. J Oncol. 2004; 9: 528-537.
- Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004; 324: 269-275.
- Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. J Nutr Metab. 2005; 2: 1-9.
- Sokoloff B, Eddy WH, Saelhof CC, Beach J. Glucose antagonists in experimental cancer. Arch Pathol Lab Med. 1955; 59: 729-732.
- Landau BR, Laszlo J, Stengle J, Burk D. Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-D-glucose. J Natl Cancer Inst. 1958; 21: 485-494.
- Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, et al. Weight-cycling decreases incidence and increases latency of mammary tumours to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-α female mice. Cancer Epidemiol Biomarkers Prev. 2002; 11: 836-843.
- Meadows GG, Fu YM, Meadows GG, Fu YM. Dietary restriction of specific amino acids modulates tumor and host interactions. Med Oncol. 2005; 271-283.