Case Report - Onkologia i Radioterapia ( 2023) Volume 17, Issue 3
Efficacy of VMAT-lattice Spatially Fractionated Radiation Therapy (SFRT) for the treatment of large abdominal sarcoma
Angel Montero1*, Alejandro Prado2, Raquel Ciervide1, Mercedes Lopez1, Beatriz �lvarez1, Emilio Sanchez1, Ovidio Hernando1, Mariola Garc�a-Aranda1, Xin Chen1, Jeannete Valero1, Rosa Alonso1, Pedro Fernandez-Leton2 and Carmen Rubio12Department of Medical Physics, HM Hospitals, Madrid, Spain
Angel Montero, Department of Radiation Oncology, HM Hospitals, Madrid, Spain, Email: angel.monteroluis@gmail.com
Received: 22-Feb-2023, Manuscript No. OAR-23-89889; Accepted: 19-Mar-2023, Pre QC No. OAR-23-89889 (PQ); Editor assigned: 24-Feb-2023, Pre QC No. OAR-23-89889 (PQ); Reviewed: 09-Mar-2023, QC No. OAR-23-89889 (Q); Revised: 17-Mar-2023, Manuscript No. OAR-23-89889 (R); Published: 24-Mar-2023
Abstract
The treatment of abdominal sarcomas represents a tough challenge because of their large size at diagnosis on many occasions and because of the limited effectiveness of available therapeutic options. Preoperative radiotherapy has proven to be a useful treatment for sarcomas and facilitates, due to its cytoreductive effect, the possibility of performing extensive surgery with radical intent in patients who would otherwise not be candidates for it. Spatially Fractionated Radiation Therapy (SFRT) lattice is an irradiation modality that, through the administration of very high doses geometrically delivered in specific areas of the tumor, aims to maximize the physical, biological, and immunomodulatory effects of radiotherapy to reduce the tumor burden, facilitate surgery and improve the results. We present a single clinical case of a patient with a giant gastric leiomyosarcoma successfully managed with lattice VMAT, external beam radiotherapy, and surgery.
Keywords
bone metastases, cardiovascular disease, prostate cancer, serum prostatic acid phosphatase, serum prostatic acid phosphatase
Introduction
Optimal treatment for large retroperitoneal tumors represents a challenge for radiation therapy since conventional-dose RT and chemo radiation are frequently limited in the ability to deliver the planned Tumor-toxic doses to Gross Tumor Volumes (GTVs). Spatially Fractionated Radiation Therapy (SFRT) is a type of radiation therapy that delivers multiple, non-overlapping beams of radiation to a target area, resulting in a spatial distribution of dose. SFRT is designed to improve the delivery of radiation therapy to the target volume while minimizing exposure to surrounding healthy tissues. In addition, SFRT has the potential to stimulate the immune response against tumor cells [1-3].
Lattice technique is a type of SFRT that uses a lattice or grid-like device to deliver high doses of radiation to a selected macroscopic tumor area. The lattice helps to shape the radiation beam, ensuring that the highest radiation dose reaches the target area, and the lowest dose reaches surrounding healthy tissue. Lattice SFRT with Volumetric Modulated Arc Therapy (VMAT) uses a combination of a virtual lattice and VMAT technology to deliver high-precision, conformal radiation therapy to the target area. Lattice VMAT has found promising results in the treatment of soft-tissue and bone sarcoma, although evidence of efficacy for retroperitoneal sarcoma is scarce. We present a clinical case of voluminous abdominal sarcoma successfully treated with lattice VMAT and review the existing evidence in the scientific literature supporting its use.
Materials and Methods
Case report
A 77-year-old male with no previous diseases of interest reported marked weight loss during the last 3 months. A palpable mass of more than 20 cm was located in his left upper abdomen. 18 FDGPET-CT-scan showed an irregular mass measuring 20×19×13 cm that appeared to be dependent on the gastric wall of the fundus and greater curvature with SUV (Standardized Uptake Values) max 19.03 and extending caudally to reach the pelvis (Figure 1a). The mass included but did not infiltrate abdominal structures including the spleen, kidney and left adrenal gland, and mesenteric artery trunk. A core biopsy confirmed a fuso-cellular neoplasm with intense atypia and muscular differentiation compatible with pleomorphic leiomyosarcoma. The patient underwent four courses of systemic treatment with docetaxel and gemcitabine without radiologic response. The tumor was considered unresectable in an institutional multidisciplinary tumor board and was remitted to our department to consider radiation treatment (Figure 1b).
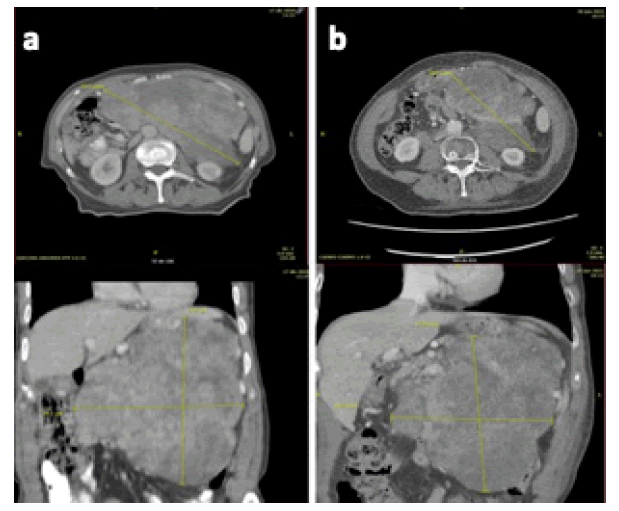
Figure 1:a. CT images of gastric leiomyosarcoma pre-lattice radiotherapy and b. 6 months after treatment previous to surgical resection (b
Radiation therapy
Patient immobilization was performed by using a vacuum cushion routinely utilized in our department for most abdominal VMAT treatments. Axial images with a thickness of 3 mm were obtained from the lung tips and extended below the obturator holes. Volumes of interest were defined by using the Ray Station planning system (Ray Search Laboratories, Stockholm, Sweden). Gross Tumor Volume (GTV) was defined as the macroscopically visible tumor observed on the axial images and Clinical Target Volume (CTV) was determined by adding 1 cm in cephalocaudal and 0.5 cm in radially direction to GTV. Planning Target Volume (PTV) was defined by adding 0.5 cm to CTV. Total volumes for CTV and PTV were 2387 cc and 3863 cc, respectively. The Organ at Risk (OAR) including the spinal cord, right kidney, and small and large bowel was contoured and classified as avoidance structures. Lattice VMAT consisted of two different steps: an initial fractionated VMAT with 40 Gy delivered over 4 weeks in 20 fractions of 2 Gy with 4 VMAT arcs using 6MV FlatteningFilter Free (FFF) energy beams, daily IGRT (Image-Guided Radiation Therapy) verification using LINAC-kV-cone-beam CT and SGRT (Surface-Guided Radiation Therapy) based on the Catalyst™ system (C-RAD AB, Uppsala, Sweden) and using the Hexa POD 6D system for rotational corrections (Elekta AB, Stockholm, Sweden), followed the day after by a single fraction SFRT of 18 Gy.
Clinical design of VMAT lattice comprised of high-dose regions shaped as 1 cm spheres placed inside the GTV. Exact sphere positions were determined by medical physicist and radiation oncologist according to GTV shape and its proximity to OAR. Lattice geometry comprised 17 high-dose regions shaped as 1.5 cm diameter spheres. These spheres were placed inside the lattice volume (GTVlattice), defined as a 2 cm subtraction from the PTV, to minimize the dose to OAR. High-dose spheres were located in different planes, being 5 cm the average centreto-centre separation between neighbouring spheres (Figure 2a). For each sphere 95% of the prescribed peak dose (18 Gy) should cover 95% of the volume. The maximum dose allowed inside each sphere was 23 Gy. The valley dose should be kept as low as reasonable achievable (ideally below 5 Gy). The Valley-To-Peak Dose Ratio (VPDR) was calculated as the average dose of the 5% of GTVlattice which received the lowest dose (Davg (95-100)) divided by the prescribed Peak Dose (Dp) as previously described [4]. The peripheral dose, defined as the D99% of GTVlattice, was also obtained. The following dose constraints and prescription details were used: 95% of prescription should be covered 95% PTV; 105% of prescription dosage could cover a maximum of 5% PTV; heart: D5cc>21Gy; lungs: V16.7<10%; duodenum and small bowel: V16.5<5cc; liver: V15<50%, V21<30%; right kidney (contralateral): V12<33%; colon: max point dose <28.2 Gy, V24<20cc; spinal cord: max point dose <18 Gy (Fig. 2b). The average sphere D95% was 17.7 Gy ± 0.3Gy, the average sphere mean dose value was 19.6 Gy ± 0.4 Gy and the average sphere D1% was 21.6±0.7 Gy. Doses between neighbouring spheres ranged between 6 Gy and 8 Gy, respectively. VPDR obtained was 0.3 Gy and the peripheral dose was 1.7 Gy. The QA verification results were 98.2% and 99.6% for the measurements obtained with 1500 and SRS1000 detectors, respectively (Figure 2b).
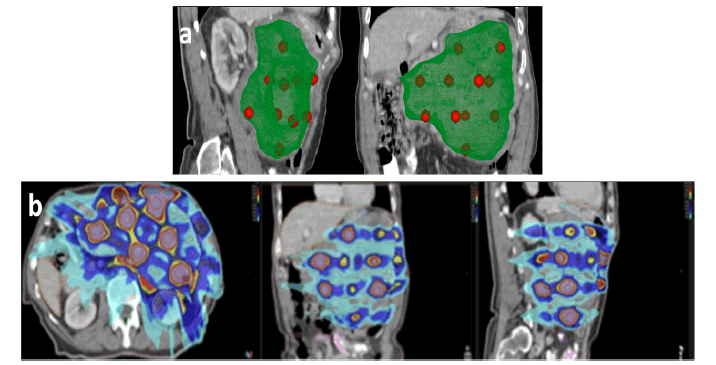
Figure 2: 2a. Dosimetric distribution for virtual VMAT lattice: a) 1 cm spheres (red) into de GTV (green) b. dose gradient with VMAT lattice from 18 Gy (yellow) and 21 Gy (brown) in vertices to 8 Gy (blue) and 4.5 Gy (burgundy) in valley
Treatment results
The patient completed the treatment course without unplanned interruptions. On follow-up, only grade 1 acute fatigue was reported. Evaluation by 18-FDG-PET-CT 6 months after the end of lattice VMAT showed a reduction in tumor volume, measured according to the CTV variation before and after irradiation, of 32% (from 2387cc to 1621cc) and, more importantly, regained the possibility of performing a complete resection (Figure 1b). Eight months after the end of lattice VMAT the patient underwent surgery and a complete R0 excision with negative margins of the abdominal tumor was performed, showing on pathologic exam a grade 2 pleomorphic leiomyosarcoma with the presence of 30% necrosis and 5 mitoses per 10 HPF. At the last visit 24 months after the end of irradiation, the patient is stable and in good condition with no signs of local or distant relapse, and without the appearance of late complications attributable to the treatment. The main benefit obtained with the VMAT lattice treatment was to make resectable a disease considered at diagnosis as unresectable due to its size, location, and involvement of other structures.
Discussion
Lattice radiotherapy intends to deliver inhomogeneous high doses to different areas within the tumor by directing small, precise beams of radiation at a cancerous area from different angles. The spatial fractionation of the radiation dose results in the creation of regions of high dose within the target area, as well as areas of lower dose surrounding it. This allows for more precise delivery of radiation to the target area, with less exposure to surrounding healthy tissues, which can help to reduce side effects and increase the effectiveness of the therapy [4,5].
The main radiobiological mechanisms related to lattice SFRT involve radiation-induced non-targeted direct effects, microvascular alterations, and immunomodulation. Non-targeted radiation effects are classified into bystander, cohort, and abscopal effects. Bystander effects are defined as radiation-induced, signal-mediated effects in uneradicated cells adjacent to a target volume that is exposed to very low levels of scatter radiation. Cohort effects are the response in irradiated cells that are not a consequence of direct energy deposition in the target cell, but due to the communication between cells within an irradiated volume and are based on intercellular signalling. The abscopal effect is an immune-mediated response of distant lesions secondary to immunogenicity.
In radiotherapy, cells exposed to high doses of ionizing radiation undergo an increase in apoptosis due to cell signalling, either by direct physical contact or cell-released cytokines, nitric oxide, or reactive oxygen species. For the lattice radiation treatment, most of the benefits come from the destruction of volumes receiving the highest dose of radiation while inducing bystander effects in those cells in peripheral zones well within the target but farther away from healthy adjacent structures. In large solid tumors, extensive areas are poorly vascularized, which leads to volumes with low oxygen concentration, a condition that promotes tumor survival via upregulation of Hypoxia-Inducible Factor 2 Alpha (HIF-2). High-dose radiation causes endothelial apoptosis via the production of soluble factors and alters tumor microvasculature which is crucial for local tumor growth and metastatic dissemination. Finally, lattice SFRT works by damaging the DNA of cancer cells, which can lead to cell death or inhibit their ability to divide and grow.
Different studies have shown that lattice SFRT can induce an immune response against cancer cells, which can help to slow or stop their growth and spread. This is thought to occur because lattice SFRT can cause cancer cells to release antigens, which are substances that can trigger an immune response. In addition, lattice SFRT may stimulate immune cells, such as T cells and natural killer cells, which can help to attack and destroy cancer cells. Likewise, some studies have also suggested that lattice SFRT may enhance the effects of immunotherapy and stimulate the immune response against tumor cells by creating an immunogenic response, also known as an "abscopal effect." This is a phenomenon where radiation therapy not only destroys the cancer cells within the treated area but also stimulates the immune system to attack cancer cells at distant sites. The exact mechanisms by which lattice radiation stimulates the immune response against tumor cells are not fully understood, but it is believed to involve the release of antigens and Damage-Associated Molecular Patterns (DAMPs) from the cancer cells that are targeted by the radiation. These antigens and DAMPs can stimulate the immune system to recognize and attack cancer cells, both in the treated area and at distant sites. It is important to note that the immunogenic response to lattice radiation is not seen in all patients and the exact mechanisms and factors that contribute to this response are still being studied [2,3]. More research is needed to fully understand the relationship between lattice radiation and the immune response against tumor cells, but the potential for this treatment to stimulate the immune system provides an exciting area of investigation in the field of cancer treatment.
The management of STS has been immersed in continuous evolution. Randomized studies settled that the addition of radiotherapy either before (preoperative) or after (postoperative) to the surgical procedure allowed more conservative resections while improving local control. The probability of tumor control growth as radiation dose increases, favouring the eradication of more resistant tumor clones. Even in patients considered to be at low risk of local recurrence, doses above 60 Gy are predictors of local control, an advantage that is accentuated in high-risk patients (i.e., tumors> 3cm, affected resection borders, recurrent tumors) with doses greater than 65 Gy [6-9]. Studies have shown that lattice radiotherapy is effective in delivering high doses of radiation to sarcomas while minimizing exposure to surrounding healthy tissues favouring dose escalation. Table 1 resumes published results of studies with different lattice techniques for sarcoma. Most of them are reports of few clinical cases, and only one study exceeds thirty cases, in which lattice radiotherapy was administered prior to conventional irradiation. The lattice techniques employed included the fabrication of collimator blocks, using a multi-leaf collimator and, in 2 studies, using a virtual VMAT lattice [10]. Reported results show acceptable tolerance to the technique and pathologic complete response rates between 26% and 100% of the cases that underwent subsequent surgery.
Tab. 1. Resume of studies with lattice radiotherapy for sarcoma
| Author/year | N | Tumor location | Latticesequence | GRID/Latticedose (Gy) | EBRT dose (Gy) | Tumor response | Adverse effects | |
|---|---|---|---|---|---|---|---|---|
| Mohiuddin (2009) [9] | 33 | Diverse | pre-EBRT | 20-Dec | 22-70 | Collimator block lead | pCR: 26% | Mild acute and late toxicities; 2 Grade 3 acute skin reaction |
| pPR: 50% | ||||||||
| Kaiser (2013) [10] | 1 | Limb | Inter EBRT | 18 | 32 | Collimator block lead | pCR: 100% | No skin toxicity |
| Mohiuddin (2014) [11] | 14 | Diverse | pre-EBRT | 18 | 50 | Collimator block lead | pCR: 65% | 1 Grade 3 acute skin reaction; 2 late wound healing |
| Grams (2020) [12] | 1 | Abdomen | pre-EBRT | 18 | 30 | VMAT | marked reduction in tumor size; symptomatic relief | NR |
| Snider (2020) [13] | 26 | Diverse | pre-EBRT | 15 | 40-50.4 | Multileafcollimator | pCR: 35.3% | 27% > Grade 3 acute skin toxicity |
| Tajiki (2021) [14] | 1 | Limb | pre-EBRT | 15 | 50 | Collimator block lead | PR >50% | NR |
| Borzov(2022) [15] | 3 | Limb | pre-EBRT | 20 | 50 | VMAT | pCR: 66% | G≥2: 0 |
| Current case | 1 | Abdomen | post-EBRT | 18 | 40 | VMAT | Pre-surgical reduction >25% | Fatigue G1 |
There is a growing body of evidence supporting the efficacy of VMAT lattice that is capable of delivering high doses of radiation to the tumor in significantly shorter time periods compared to 3D-CRT and IMRT [11,12]. The improved delivery time of VMAT lattice can have several benefits for patients, including reduced exposure to ionizing radiation and improved comfort during treatment. In addition to the improved targeting of the tumor, the real-time modulation of the radiation beam in VMAT lattice also allows for improved delivery of the radiation dose, which can help to enhance the therapeutic effects of the treatment. Finally, the ability to deliver high doses of radiation more quickly can also help to improve the overall efficiency of the radiation therapy process, potentially allowing for more patients to be treated in a shorter time period. By contrast, VMAT lattice also has some limitations to consider VMAT lattice requires specialized equipment, including a linear accelerator capable of delivering volumetric modulated arc therapy, and software capable of generating and delivering complex treatment plans; VMAT lattice treatment plans can be complex and time-consuming to generate, requiring expertise in treatment planning; finally, there is still limited clinical data evaluating the long-term outcomes of VMAT lattice for the treatment of cancer, and further studies are needed to fully understand its efficacy and limitations [13-15].
Conclusion
In conclusion, the biological basis for the use of VMAT lattice for voluminous retroperitoneal tumors lies in its ability to deliver high doses of radiation to the tumor. These high doses administered as a single fraction make it possible to exploit the immunogenic potential of lattice radiotherapy and contribute not only to a physical but also to a biological scaling of radiation treatment. The limited evidence suggests that VMAT lattice may be a promising option for sarcomas, with improved delivery times and reduced exposure to surrounding healthy tissues compared to other radiation therapy techniques. However, further studies are needed to fully evaluate its efficacy for this type of cancer.
References
- Billena C, Khan AJ. A current review of spatial fractionation: Back to the future? Int. J. Radiat. Oncol. Biol. Phys. 2019; 104:177-87.
- Pellizzon AC. Lattice radiation therapy–its concept and impact in the immunomodulation cancer treatment era. J. Braz. Med. Assoc. 2020; 66:728-31.
- Moghaddasi L, Reid P, Bezak E, Marcu LG. Radiobiological and Treatment-Related Aspects of Spatially Fractionated Radiotherapy. Int. J. Mol. Sci. 2022; 23:3366.
- Wu X, Perez NC, Zheng Y, Li X, Jiang L, et al. The technical and clinical implementation of LATTICE radiation therapy (LRT). Radiat. Res. 2020; 194:737-46.
- Duriseti S, Kavanaugh J, Goddu S, Price A, Knutson N, et al. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv. Radiat. Oncol. 2021; 6:100639.
- Jebsen NL, Trovik CS, Bauer HC, Rydholm A, Monge OR, et al. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: a Scandinavian sarcoma group study. Int. J. Radiat. Oncol. Biol. Phys. 2008; 71:1196-203.
- Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010; 77:203-9.
- Jacobs AJ, Michels R, Stein J, Levin AS. Improvement in overall survival from extremity soft tissue sarcoma over twenty years. Sarcoma. 2015.
- Mohiuddin M, Miller T, Ronjon P, Malik U. Spatially fractionated grid radiation (SFGRT): A novel approach in the management of recurrent and unresectable soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2009;75: S526.
- Kaiser A, Mohiuddin MM, Jackson GL. Dramatic response from neoadjuvant, spatially fractionated GRID radiotherapy (SFGRT) for large, high-grade extremity sarcoma. J. Radiat. Oncol. 2013; 2:103-6.
- Mohiuddin M, Memon M, Nobah A, Elsebaie M, AL Suhaibani A, et al. Locally advanced high-grade extremity soft tissue sarcoma: Response with novel approach to neoadjuvant chemoradiation using induction spatially fractionated GRID radiotherapy (SFGRT).
- Grams MP, Owen D, Park SS, Petersen IA, Haddock MG, et al. VMAT GRID therapy: a widely applicable planning approach. Pract. Radiat. Oncol. 2021;11: e339-47.
- Snider JW, Molitoris J, Shyu S, Diwanji T, Rice S, et al. Spatially fractionated radiotherapy (GRID) prior to standard neoadjuvant conventionally fractionated radiotherapy for bulky, high-risk soft tissue and osteosarcomas: Feasibility, safety, and promising pathologic response rates. Radiat. res. 2020; 194:707-14.
- Tajiki S, Gholami S, Kazemian A, Haddad P, Esfahani M, et al. Management of bulky high-grade pleomorphic sarcoma using grid therapy technique. Int. J. Radiat. Res. 2021; 19:239-42.
- Borzov E, Bar-Deroma R, Lutsyk M. Physical aspects of a spatially fractionated radiotherapy technique for large soft tissue sarcomas. Phys. Imaging Radiat. Oncol. 2022; 22:63-6.



