Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 12
Efficacy and Tolerability of CDK4/6 Inhibitors in Patients with HR+/HER2âËâ Metastatic Breast Cancer: Real-World Data
Abir Oufrid1*, Basma Aabboub2, Diango Keita3, Sara Nejjari4, Samia El Hakym5, Hafssa El Hilali6, Chaymae Chbihi7, Lamiae Amaadour8, Karima Oualla9, Zineb Benbrahim10, Samia Arifi11 and Nawfel Mellas122Department of Medical Oncology, CHU Hassan II, Fès, Morocco
3Department of Medical Oncology, CHU Hassan II, Fès, Morocco
4Department of Medical Oncology, CHU Hassan II, Fès, Morocco
5Department of Medical Oncology, CHU Hassan II, Fès, Morocco
6Department of Medical Oncology, CHU Hassan II, Fès, Morocco
7Department of Medical Oncology, CHU Hassan II, Fès, Morocco
8Department of Medical Oncology, CHU Hassan II, Fès, Morocco
9Department of Medical Oncology, CHU Hassan II, Fès, Morocco
10Department of Medical Oncology, CHU Hassan II, Fès, Morocco
11Department of Medical Oncology, CHU Hassan II, Fès, Morocco
12Department of Medical Oncology, CHU Hassan II, Fès, Morocco
Abir Oufrid, Department of Medical Oncology, CHU Hassan II, Fès, Morocco, Email: abir.oufrid@usmba.ac.ma
Received: 01-Dec-2025, Manuscript No. OAR-25-177745; , Pre QC No. OAR-25-177745 (PQ); Editor assigned: 03-Dec-2025, Pre QC No. OAR-25-177745 (PQ); Reviewed: 19-Dec-2025, QC No. OAR-25-177745; Revised: 24-Dec-2025, Manuscript No. OAR-25-177745 (R); Published: 31-Dec-2025
Abstract
Introduction: Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, in combination with hormone therapy, are currently the standard treatment for hormone receptor–positive, HER2-negative (HR+/HER2−) metastatic breast cancer. Although their efficacy has been demonstrated in randomized clinical trials, real-world data remain limited, particularly in populations underrepresented in pivotal studies. The objective of this study was to evaluate, in a real-world setting, the efficacy and Tolerability of CDK4/6 inhibitors in patients with HR+/HER2− metastatic breast cancer. Methods: This was a retrospective study of patients admitted to the medical oncology department at CHU HASSAN II Hospital in Fès, who received CDK4/6 inhibitors for HR+/HER2− metastatic breast cancer over a six- Year period, from 2018 to 2023, regardless of the date of their first consultation.
Results: Thirty-two patients were included in our study, of whom 54% were postmenopausal at diagnosis. The mean age was 52.42 years (range: 32–93), 19% had a family history of cancer, and 81% had an ECOG Performance status of 1. The most frequent metastatic site was bone, observed in 75% of patients— solitary in 6% and associated with other sites in 69%. Prior treatments had been administered to 56% of patients, including radical mastectomy (68%), adjuvant chemotherapy (28%), radiotherapy (62%), or adjuvant hormone therapy (100%). The hormone therapy administered was based on palbociclib and letrozole. CDK4/6 inhibitors were used as first-line therapy in 79% of patients and after prior Treatment in 21%. The mean follow-up duration was 38.4 months, with a mean progression-free Survival of 20.88 months. Neutropenia was the most frequently observed adverse event, followed by fatigue, anemia, rash, and thrombocytopenia. Neutropenia and thrombocytopenia were the most Severe side effects.
Conclusion: In routine clinical practice, CDK4/6 inhibitors combined with hormone therapy demonstrate significant clinical efficacy and an acceptable tolerability profile in patients with HR+/HER2− metastatic breast cancer. These results confirm the translatability of clinical trial data to real-world settings and support the use of these treatments as a therapeutic cornerstone in this indication.
Keywords
Metastatic Breast Cancer; Hormone Receptor–Positive; Her2-Negative; Cdk4/6 Inhibitors; Real-World Data; Hormone Therapy
INTRODUCTION
Hormone receptor–positive, HER2-negative (HR+/HER2−) metastatic breast cancer represents the most common subtype of metastatic breast cancer. For many years, hormone therapy was the standard treatment, but its efficacy was limited by the almost inevitable development of resistance [1]. The identification of the key role of the cyclin D–CDK4/6 complex in cell cycle regulation led to the development of CDK4/6 inhibitors, including palbociclib, ribociclib, and abemaciclib. When combined with hormone therapy, these agents have demonstrated a significant improvement in
Progression-free survival and, for some, overall survival in several phase III randomized clinical trials, such as PALOMA-2, MONALEESA-2, and MONARCH-3 [2-3, 4]. These results led to their adoption as standard first-line treatment for HR+/HER2− metastatic breast cancer. However, clinical trials enroll
Selected populations that do not always reflect the diversity of patients treated in routine clinical practice. Real-world data are therefore essential to evaluate the efficacy and tolerability of CDK4/6 inhibitors under everyday care conditions [5]. The objective of this study was to assess, in a real-world setting, the efficacy and tolerability of CDK4/6 inhibitors in patients with HR+/HER2− metastatic breast cancer.
Materials and Methods
Study design and duration
This retrospective study included patients treated with CDK4/6 inhibitors for HR+/HER2− metastatic breast cancer at the Medical Oncology Department of CHU Hassan II, Fès, Morocco, over a six-year period from 2018 to 2023.
Inclusion criteria
Patients were eligible if they were histologically confirmed invasive breast cancer, HR+ status (ER+ and PR+), HER2-negative by immunohistochemistry or FISH, and they were radiologically confirmed metastatic disease
Exclusion criteria
Exclusion criteria included Non-metastatic breast cancer or HR− or HER2+ metastatic breast cancer
Data collection
Demographic, clinical, biological, and therapeutic data were extracted from medical records and entered into an anonymized Excel database with unique patient codes. Variables included age, menopausal status, ECOG performance status, prior therapies, metastatic sites, treatment line, endocrine therapy type, and adverse events.
Endpoints
- Primary endpoint: Progression-free survival (PFS), defined as time from CDK4/6 initiation to disease progression or death.
- Secondary endpoints: Overall survival (OS), defined as time from treatment initiation to death from any cause; and tolerability, assessed according to CTCAE v5.0, including dose modifications and treatment discontinuation.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained from the local Ethics Committee. Patient confidentiality was maintained through anonymization.
Statistical analysis
Data were analyzed using SPSS v25.0. Qualitative variables were expressed as counts and percentages; quantitative variables as mean ± SD or median with range. Kaplan–Meier estimates were used for PFS and OS curves; comparisons between subgroups were performed using the log-rank test. Statistical significance was set at p < 0.05.
Results
Baseline patient characteristics
A total of 32 patients were included in the study. The mean age was 52.4 years (range: 32–93 years), and 54% of patients were postmenopausal at the time of diagnosis. Nineteen percent had a family history of cancer, and 81% had an ECOG performance status of 1. The most frequent metastatic site was bone, observed in 75% of patients. Bone involvement was isolated in 6% of cases and associated with other metastatic sites in 69% of cases. Among the patients, 56% had received prior treatment, including radical mastectomy (68%), adjuvant chemotherapy (28%), radiotherapy (62%), and adjuvant endocrine therapy (100%). The endocrine therapy administered in this study consisted of palbociclib plus letrozole. CDK4/6 inhibitors were used as first-line therapy in 79% of patients and in subsequent lines in 21% [Table 1].
| Characteristics | N (%) or mean +/-standard deviation |
|---|---|
| “Demographic characteristics” | |
| Age (years) | 52,4 (32-93) |
| Menopausal status | 17 (54%) |
| Family history of cancer | 6 (19%) |
| ECOG performance status 1 | 26 (81%) |
| Metastatic sites | |
| Isolated bone | 2 (6 %) |
| Bone + other sites | 22 (69 %) |
| Other sites (visceral/lymph node) | 8 (25 %) |
| Prior treatments | |
| Radical mastectomy | 19 (68 %) |
| Adjuvant chemotherapy | 8 (28 %) |
| Radiotherapy | 14 (62 %) |
| Adjuvant endocrine therapy | 32 (100 %) |
| CDK4/6 inhibitor therapy | |
| Line of treatment | |
| First-line | 25 (79 %) |
| Subsequent lines | 7 (21 %) |
| Type of associated endocrine therapy | Palbociclib + Letrozole (100 %) |
Table 1: Baseline patient characteristics
Efficacy
Median follow-up was 38.4 months. Median PFS was 20.88 months. The majority of patients received first-line CDK4/6 inhibitors (79%), which may explain the prolonged disease control observed. These results confirm the efficacy of CDK4/6 inhibitors combined with endocrine therapy in real-world practice for HR+/HER2− metastatic breast cancer.
Tolerability
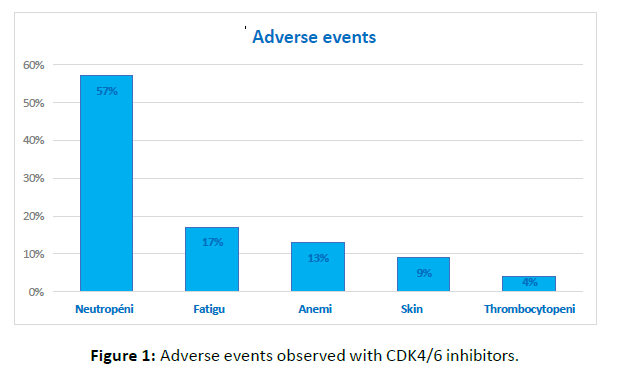
CDK4/6 inhibitor therapy combined with endocrine therapy was generally well tolerated in our series. The most frequently observed adverse event was neutropenia, reported in 57% of patients. Among these, 65% had grade III neutropenia, while 35% had grade II neutropenia. Neutropenia was the most severe hematologic adverse event observed. Asthenia was reported in 17% of patients, followed by anemia in 13%. Less frequent non-hematologic adverse events included rash in 9% of patients and thrombocytopenia in 4% (Figure 1). No unexpected adverse events were reported during follow-up.
Figure 1: Adverse events observed with CDK4/6 inhibitors.
DISCUSSION
In our retrospective study involving 32 patients with HR+/HER2− metastatic breast cancer treated with CDK4/6 inhibitors combined with hormone therapy, we observed a median progression-free survival (PFS) of 20.88 months, with an overall acceptable tolerability. This PFS duration is generally consistent with the benefits reported in pivotal clinical trials and real-world studies, while reflecting the specific characteristics of a routine clinical practice population. CDK4/6 inhibitors have profoundly changed the therapeutic paradigm of HR+/HER2− metastatic breast cancer. In the PALOMA-2 trial, the addition of palbociclib to hormone therapy significantly improved median PFS (27.6 months vs. 14.5 months), demonstrating a substantial benefit compared to hormone therapy alone [2]. Similarly, the MONALEESA-2 and MONARCH-3 trials confirmed the efficacy of ribociclib and abemaciclib, respectively, in the first-line setting, with comparable gains in PFS [3, 4]. These trials established these agents as standard first-line treatment for this patient population. In our cohort, the observed mean PFS is slightly lower than that reported in pivotal trials, which is expected in a real-world context where patients generally present a broader range of clinical characteristics and may receive these therapies in later lines. A systematic review of real-world evidence shows that CDK4/6 inhibitors are associated with improvements in PFS and other clinical outcomes, although results are heterogeneous depending on the population, treatment regimens, and follow-up durations [6]. Moreover, a recent meta-analysis integrating phase III randomized trials demonstrated that adding a CDK4/6 inhibitor to hormone therapy improves not only PFS but also other clinically relevant outcomes, such as time to subsequent progression and combined overall survival rates [7]. These data reinforce the importance of these agents in the modern therapeutic strategy for metastatic breast cancer. Clinically, administering CDK4/6 inhibitors in the first-line setting, as in our series, aligns with current recommendations and reflects established evidence-based practice. The benefits observed in our cohort, although slightly more modest, confirm that the clinical efficacy of CDK4/6 inhibitors is maintained outside the strict setting of clinical trials, particularly in more heterogeneous populations or those with more frequent comorbidities. Regarding tolerability, the profile observed in our study is consistent with the literature: neutropenia remains the most common adverse event, with a high incidence but generally manageable and severe hematologic events remain rare [8]. Other adverse events, such as fatigue and anemia, were less frequent in our series, reflecting the variability seen in real-world data [2-5, 8,9].
Finally, although our study did not allow for a robust evaluation of overall survival, several pivotal trials, such as MONALEESA-2, have already shown a significant overall survival benefit with certain CDK4/6 inhibitor plus hormone therapy combinations [3]. These results support ongoing evaluation of this endpoint in future extended follow-up of real-world cohorts. This study does have important limitations related to its retrospective nature, small sample size, and the absence of precise temporal data allowing for individual survival curve generation. Nevertheless, our results provide a valuable contribution to real-world data on the use of CDK4/6 inhibitors in HR+/HER2− metastatic breast cancer, complementing evidence from randomized controlled trials.
CONCLUSION
In our series of patients with HR+/HER2− metastatic breast cancer, the use of CDK4/6 inhibitors in combination with hormone therapy demonstrated significant clinical efficacy in real-world conditions, with a mean progression-free survival of 20.88 months. The observed tolerability profile was acceptable, mainly characterized by manageable hematologic events, particularly neutropenia. These results confirm the translatability of data from pivotal clinical trials (PALOMA-2, MONALEESA-2, and MONARCH-3) to routine practice and underscore the central role of CDK4/6 inhibitors as a first-line therapeutic cornerstone for HR+/HER2− patients. Despite the inherent limitations of its retrospective and single-center design, this study provides valuable local data on the efficacy and tolerability of these treatments, thus contributing to the growing body of real-world evidence. Finally, these observations reinforce the importance of early and personalized management and support prospective follow-up to better assess the impact on overall survival and quality of life in routine clinical practice.
Conflicts of Interest
The authors declare no conflicts of interest related to the publication of this article.
References
- Rugo HS, et Endocrine therapy for hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2016; 34: 3069-3076. [Crossref], [Google Scholar], [PubMed]
- Finn RS, et Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375: 1925-1936. (PALOMA-2) [Crossref], [Google Scholar], [PubMed]
- Hortobagyi GN, et Ribociclib as first-line therapy for HR-positive advanced breast cancer. N Engl J Med. 2016; 375: 1738-1748. [Crossref], [Google Scholar], [PubMed]
- Goetz MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017; 35: 3638-3646. [Crossref], [Google Scholar], [PubMed]
- Harbeck Real-world evidence of CDK4/6 inhibitors in HR+/HER2- metastatic breast cancer. ESMO Open. 2020; 5: e000743. [Crossref], [Google Scholar], [PubMed]
- Abdolkarimi B, Panahi N (2025) Building Therapeutic Nature Ecotourism Camps for Cancer Patients in Iran A New Approach toHealth Tourism Focusing on Lorestan Province. Int. J. Health Sci. Biomed. 2: 1-4. [Google Scholar]
- Patel. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic review of real-world evidence studies. [Google Scholar]
- Meta-analysis of CDK4/6 inhibitor outcomes in advanced breast cancer (PFS2, OS). [Google Scholar]
- Meta-analysis of safety data showing neutropenia and hematologic [Google Scholar]