Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 2
Diagnostic accuracy of Fine-Needle Aspiration (FNA) in comparison to Core Needle Biposy (CNB) in salivary gland lesion: A systemic review and meta-analysis
Dev Desai*Dev Desai, Department of Community Medicine, Smt. N.H.L. Municipal Medical College, Ahmedabad, Gujarat, India, Email: devhdesai01@gmail.com
Received: 04-Sep-2023, Manuscript No. OAR-24-112591; , Pre QC No. OAR-24-112591 (PQ); Editor assigned: 06-Sep-2023, Pre QC No. OAR-24-112591 (PQ); Reviewed: 20-Sep-2023, QC No. OAR-24-112591; Revised: 08-Jan-2025, Manuscript No. OAR-24-112591 (R); Published: 15-Jan-2025
Abstract
Background: Salivary gland tumors comprise 6% of total head and neck tumors. Parotid, being the major gland involved forms 80% of the cases while submandibular gland tumors make up 10-15% of the cases. However, the histopathology and cytology of these tumors are complex due to diverse patterns of growth and morphology exhibited by tumor cells, making the diagnosis a challenge. Despite being widely used as a preoperative diagnostic tool, fine needle aspiration yields results that are less than satisfactory, are time-consuming, and require local anesthesia. Most inaccurate results are due to insufficient samples. To overcome this, core needle biopsy using (Ultrasound) US guidance is used. It helps in obtaining a larger specimen, has higher sensitivity, and most importantly, can help assess the status of capsular invasion along with tumor grading. While choosing the right method of diagnosis is extremely crucial to the management of tumors, this study aims to ascertain the superior method to accelerate preoperative diagnosis and management.
Method: Detailed search through the medical literature was done using PubMed, Google Scholar, and Cochrane Library databases for the collection of relevant data. It was extracted keeping into consideration the inclusion and exclusion criteria. A total of 16 RCTs with a total of 2068 patients were selected. Meta-analysis was done using the two writers who independently assessed the caliber of each included study. The Cochrane tool was also used for bias risk apprehension. The statistical software packages RevMan (Review Manager, version 5.3), SPSS (Statistical Package for the Social Sciences, version 20), and Excel in Stata 14 were used to perform the statistical analyses.
Results: FNAC as a tool for preoperative diagnosis has a sensitivity of 93%, a specificity of 98.5%, and a positive predictive value of 0.972 in comparison to Core Needle Biposy (CNB) in the diagnosis and intervention regarding Salivary gland tumors.
Conclusion: The results suggest Core Needle Biposy (CNB) as a superior modality in the preoperative diagnosis of salivary gland tumors as compared to FNAC. However, FNAC can be used as a preliminary step in the process of diagnosis. Nevertheless, CNB overcomes the shortcomings of FNAC, making it a better method.
Keywords
FNA; CNB; Salivary gland tumors; Meta-analysis
Introduction
The salivary glands comprise three paired major glands (parotid, submandibular, and sublingual glands) and 600-1000 minor glands distributed widely beneath the mucosa of the oral cavity, palate, paranasal sinuses, and upper respiratory tract. Approximately 6% of all head and neck tumors occur within the salivary glands. 80% of these occur within the parotid gland, 10-15% within the submandibular gland, and the remaining 5–10% within the sublingual and minor salivary glands. Approximately 50% of submandibular gland neoplasms are benign, with pleo-morphic adenoma accounting for over a third; 50% are malignant, with adenoid cystic carcinoma being the most common, accounting for 25% of cases [1]. The histopathology and cytology of salivary gland tumors are extremely sophisticated, even for experienced pathologists, demonstrating a highly diverse mix of cell types and growth patterns and overlapping morphologic features [2-4].
Hence, the presence of a circumscribed mass or diffuse swelling of a salivary gland can represent a major diagnostic and therapeutic challenge [5]. The preoperative diagnosis of salivary gland masses is extremely important in order to avoid unnecessary surgery for nonmalignant lesions, as well as for therapeutic planning for the management of malignant tumors. Two techniques are being applied for the same.
Fine-Needle Aspiration (FNA) is a well-accepted and widely used technique for the preoperative diagnosis of salivary gland masses, with consistent specificity values of 98.5% and a sensitivity of 93% [6]. However, the results are less than satisfactory in providing a specific histopathological diagnosis and tumor grading in the case of malignancy. Although expertise in interpreting the samples is important, most inaccurate diagnoses can be attributed to suboptimal or insufficient sampling [7-9]. It also requires local anesthesia and the procedure time is somewhat longer than other modalities [10-15].
The other technique being employed, Core Needle Biopsy (CNB) under US guidance, is used frequently for masses in the head and neck area. It advocates a unique set of advantages over either palpation-guided or US-guided FNA, including the ability to obtain a larger specimen with preserved tissue architecture for possible immunohistochemical staining. This potentially contributes to the low nondiagnostic rate and high average sensitivity (92%) and specificity (100%) of this procedure, with little variation in its ability to detect malignant tumors in salivary glands [16-18]. The limitation arises cepending upon the body part that is being biopsied, as it may require general anesthesia for the entire duration of procesure or atleast anesthertic sedative and also may require monitored anesthesia care during as well as after the procedure.
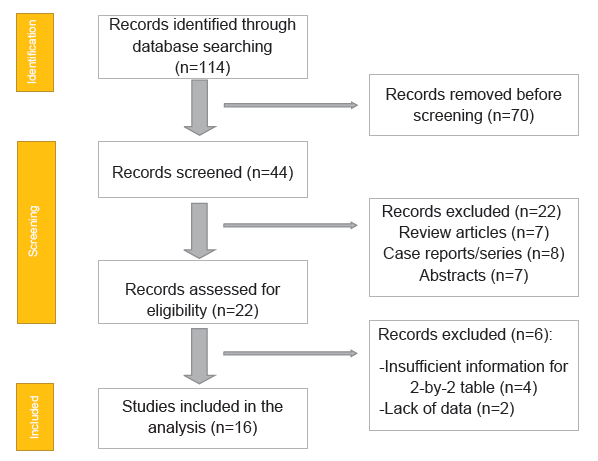
While both techniques are being employed in the health care management of salivary masses, it is important to establish the superior technique in order to ensure an appropriate, efficient, effective, and accurate diagnosis of salivary lesions that could potentially perpetuate dire consequences. This study aims to compare FNA biopsy with core needle biopsy as a diagnostic modality of salivary gland masses (Figure 1).
Fig.1. PRISMA flow chart.
Materials and Methods
Data collection
For all relevant literature, a search was done using PubMed, Google Scholar, and Cochrane Library databases. Full-text articles written only in English were considered.
The Medical Subject Headings (MeSH) and keywords ‘Core Needle Biopsy(CNB)’, ‘Fine Needle Aspiration(FNA)’, ‘salivary gland tumors’. References, reviews, and meta-analyses were scanned for additional articles.
Inclusion and exclusion criteria
Titles and abstracts were screened, and duplicates and citations were removed. References of relevant papers were reviewed for possible additional articles. Papers with detailed patient information and statically supported results were selected.
The primary objective was to determine the diagnostic accuracy of fine needle aspiration for detecting salivary gland lesions in all patients.
We included studies that compared the outcome and accuracy of Core Needle Biopsy (CNB) with Fine Needle Aspiration (FNA) for suspected salivary gland lesions in the overall population including children, Pregnant patients, and Adults.
Therefore, the purpose of this study was to perform a systematic review and meta-analysis of the use of Fine Needle Aspiration (FNA) to diagnose Salivary gland lesions in the general population, i.e., not limited to one subpopulation such as pregnant patients or children. The primary outcomes of interest are the sensitivity and specificity of FNA for this indication.
The inclusion criteria were as follows:
• Studies that included patients undergoing diagnosis on salivary glands or those having histologically verified cases.
• Prospective or retrospective studies.
• Studies that included core needle biopsy content or those comparing the diagnostic performance between FNA and CNB for salivary gland lesions.
• Studies in which data on the results of True Positives (TP), True Negatives (TN), False Positives (FP), and False Negatives (FN) were available. The exclusion criteria were: 1) Case reports, case series, abstracts, review articles. 2) Non-english language articles.
Data extraction
Each qualifying paper was independently evaluated by two reviewers. Each article was analyzed for the number of patients, age, procedure modality, and incidence of the predecided complications. Further discussion or consultation with the author and a third party were used to resolve conflicts. The study's quality was assessed using the modified Jadad score. In the end, According to PRISMA, a total of 16 RCTs with a total of 2068 patients were selected.
Assessment of study quality
Two writers independently assessed the caliber of each included study. This test consists of 10 questions, each with a score between 0 and 2, with 20 being the maximum possible overall score. Two authors rated each article independently based on the above criteria. The inter observer agreement for study selection was determined using the weighted Cohen's kappa (K) coefficient. For deciding the bias risk for RCTs, we also employed the Cochrane tool. No assumptions were made about any missing or unclear information. There was no funding involved in collecting or reviewing data.
Statistical analysis
The statistical software packages RevMan (Review Manager, version 5.3), SPSS (Statistical Package for the Social Sciences, version 20), and Excel in Stata 14 were used to perform the statistical analyses.The data was obtained and entered into analytic software [19]. Fixed or random-effects models were used to estimate sensitivity, specificity, Positive Predictive Value (PPV), Diagnostic Odds Ratios (DOR), and Relative Risk (RR) with 95 percent confidence intervals to examine critical clinical outcomes (CIs). Diagnosis accuracy and younden index were calculated for each result. Individual study sensitivity and specificity were plotted on forest plots and in the Receiver Operating Characteristic (ROC) curve. The prior odds ratio and positive and negative likelihood ratio and positive and negative post-test ratio are described in Fegan’s analysis.
Bias study
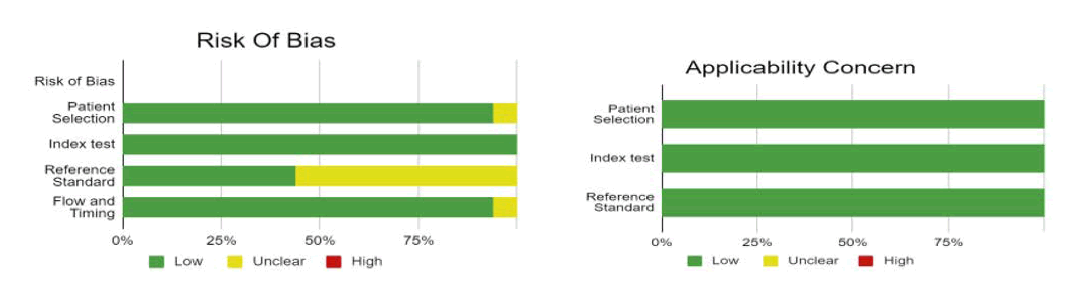
The risk of bias was evaluated by using QUADAS-2 analysis. This tool includes 4 domains as patient selection, index test, reference standard, flow of the patients, and timing of the index tests.
Results
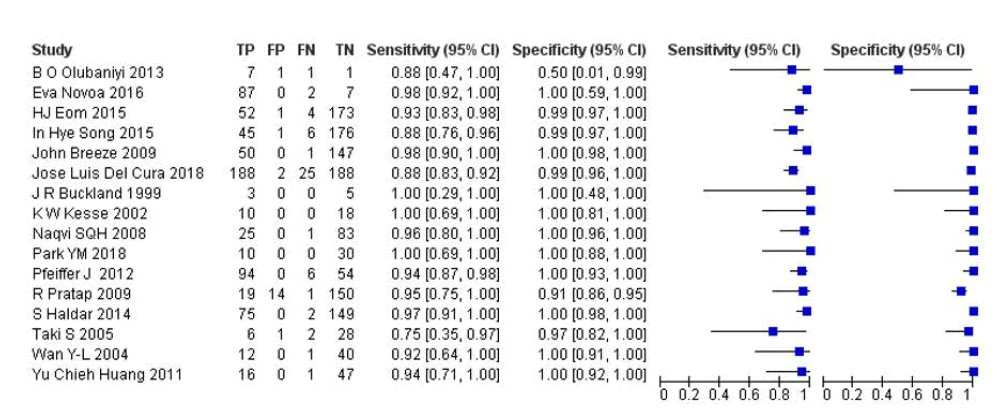
A total of 16 RCTs with 2068 patients were selected for the study (Table 1 and Figure 2).
Out of these tests, 8 tests showed a sensitivity of over 95%, and 14 tests provided a specificity of over 95%. And 7 tests showed both specificity and sensitivity over 95%. The value of true positive was 699, true negative was 1296, false negative was 53, and false positive was 20. With a confidence interval of 95%, sensitivity, specificity, and positive predictive values were calculated. A summary of this is available in Figure 2.
| Study | Location | Device | Needle size | Throw | Passes | Performed by | Site | Compression | Methodology | Complications | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Olubaniyi et al. | U.K. | Spring-loaded (Bard Magnum; C.R. Bard Inc., Covington, GA) | 18-gauge (n=8) | Variable | Avg. 2 passes (1 to 4 passes) | Radiologist | All SMG | NA | Retrospective | 0 | NA |
| Novoa et al. | Switzerland | NA | 20 gauge | Avg. 2 passes | Otolaryngologist | Parotid: 96, SMG : 14, SLG : 1 | 15 minutes | Prospective nonrandomized | 0 | 7 years, no recurrences | |
| Eom et al. | South Korea | Spring-activated | 18 gauge | 1 pass (n=155), | Radiologist | Parotid : 192, | 10-20 minutes | Retrospective | 0 | NA | |
| needles (1.1- or | 2 passes (n=95), | SMG : 65 | |||||||||
| 1.6-cm excursion; ACECUT; TSK, Tochigi, Japan) | 3 passes (n=7) | ||||||||||
| Song et al. | South Korea | Double-action, | 18 gauge | NA | Radiologist | Parotid : 171, | NA | Retrospective | 0 | 5 years (2008-2013), NA | |
| spring-activated needle (TSK ACE-CUT; Create Medic, Yokohama, Japan) | SMG : 56, SLG : 1 | ||||||||||
| J R Buckland | U.K. | Spring-loaded (Biopty Bard) | 18 gauge | 20 mm | 2 passes | 0 | |||||
| Breeze et al. | U.K. | Spring-loaded (Biopty, Bard, Helsingborg, Sweden) | 18 (n = 16); 20 (n = 38) | 22 mm (n = 16); variable 15 or 22 (n = 38) | 2-4, mean, 3 | Radiologist | Parotid : 198 | Prospective | |||
| Jose Cura | Spain | Spring-loaded (BioPince Argon Medical Devices, Plano, TX, USA) | 18 gauge | Radiologist | 3-5 minutes | Retrospective | 1 hematoma, 1 superficial soft tissue infection that did not require admission | ||||
| K W Kesse | U.K. | Biopty Gun (Bard) (n=16), Magnum Gun (Bard) (n=38) | 18 gauge (n=16), 20 gauge (n=38) | 22 mm (n = 16); variable 15 or 22 (n = 38) | 1-3, mean 2 | Parotid : 54 | Prospective | 0 | 3.5 years | ||
| Naqvi et al. | Pakistan | NA | 18 gauge | 1 pass | NA | Parotid : 70, SMG: 38 | NA | Retrospective | 2 hematomas | 3 years, NA | |
| Park et al. | South Korea | NA | NA | Radiologist | Salivary glands (not specified) | Retrospective | 0 | 2 years | |||
| Pfeiffer and Ridder | Germany | Spring-loaded | 12 (n=78), 14 (n=41), 16 gauge (n=1), NA (n=1) | Variable (15mm or 22mm) | 1.5 passes (mean 2.1) | NA | Parotid : 64, SMG : 12 | NA | Observational | 1 hematoma, 1 facial weakness due to local anesthesia | 7 years, NA |
| (Magnum Gun; Bard, Covington, GA) | |||||||||||
| Pratap et al. | U.K. | Spring-loaded, | 15 or 16 gauge (for suspected lymphoma or large lesion), 18 gauge | More than 1 pass (n=34) | NA | All parotid | NA | Retrospective | 1 hematoma | No recurrences | |
| disposable cutting needle (Temno; Bauer Medical, Clearwater, FL) | |||||||||||
| Haldar et al. | U.K. | Spring-loaded | 18 gauge (n=306), 20 gauge (n=7) | Variable (15mm-22mm) | 1 pass (n=101), 2 passes (n=165), 3 passes (n=34) | Radiologist | All parotid | Retrospective | 2 hematomas | 16 years, 2 recurrences (malignant) | |
| (Magnum Gun; Bard, Covington, GA) | |||||||||||
| Taki et al. | Japan | Spring-loaded | 18 gauge | 11mm | 2-4 passes (avg. 3 passes) | Radiologist | Parotid : 27, | NA | Retrospective | 0 | 8 years, NA |
| (ACECUT, TSK, Tochigi, Japan) | SMG : 10 | ||||||||||
| Wan et al. 2004[32] | Taiwan | Spring-loaded (Magnum, Bard; Temno) | 20 (n = 1); | 15mm | 1(n=1); 2(n=32); 3(n=17); 4 (n = 2); 5 (n = 1); mean, 2.4 | Radiologist | Parotid : 53 | 30 minutes | Retrospective | 1 hematoma | Mean 38 months, no recurrence |
| 18 (n = 20); 16 (n = 26); 14 (n = 6); mean, 16.6 | |||||||||||
| Huang et al. 2012[33] | Taiwan | Spring-loaded (Bard Magnum, Covington, GA or Temno, Carefusion Co., San Diego, CA) | 20 gauge (n=2), | 1-5 passes | NA | All parotid | 30 minutes | Retrospective | 1 hematoma (surgical approach needed) | 8 years, no recurrence | |
| 18 gauge (n=34), 16 gauge (n=26), and 14 gauge (n=5) | |||||||||||
| Note: SMG=Submandibular Gland; SLG=Sublingual Gland; Avg=Average | |||||||||||
Tab. 1. Table of the description of papers.
Fig.2. The forest plot of sensitivity and specificity.
The sensitivity of the test is 0.93 with a CI of 95% in a range of (0.898 to 0.61) the mean being (0.032). The specificity of the test is 0.985 with a CI of 95% in a range of ( 0.924 to 1.046) the mean being (0.061). The PPV is 0.972 with a CI of 95% in a range of (0.918 to 1.026) the mean being (0.054).
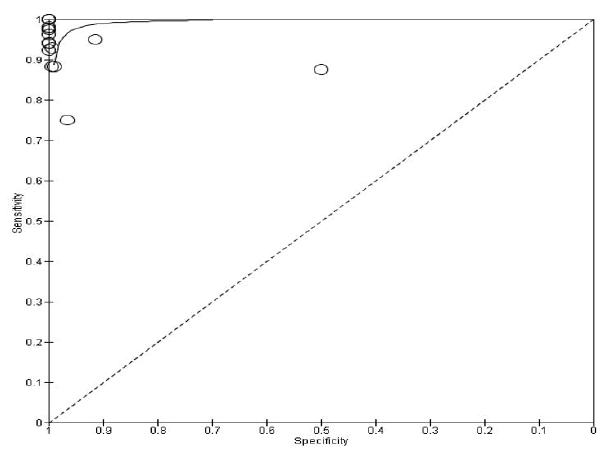
The summary of the ROC curve (Figure 3) shows that the Area Under the Curve ROC (AUC) was 0.9596. The overall Diagnostic Odds Ratio (DOR) was 854.626. Diagnostic accuracy is 0.965 and the Younden index is 0.914.
P value for Cochrane Q was 0.1817 and I2 was at 24.1%.
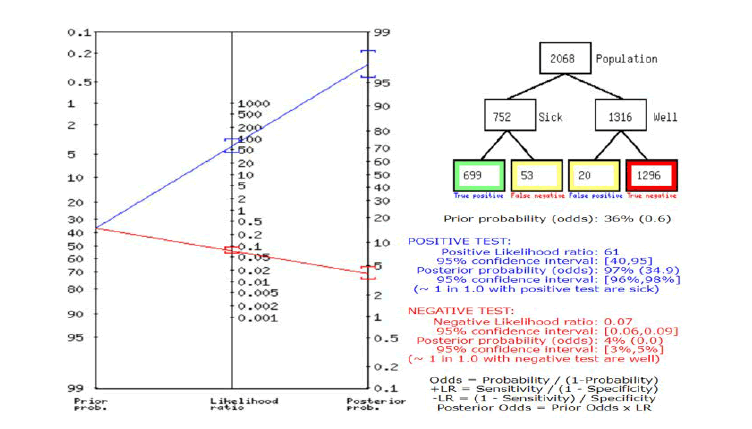
In Figure 4, a summary of Fagan’s analysis can be observed, in conclusion, the prior probability of the test was 36. The positive likelihood ratio was 61 and the post-test ratio was 97. The negative likelihood ratio was 0.07 and the post-test ratio was 4.
Fig.3. The summary of receiver operating characteristic curve for CNB vs. FNA scan.
Fig.4. Fagan’s nomogram.
Bias study
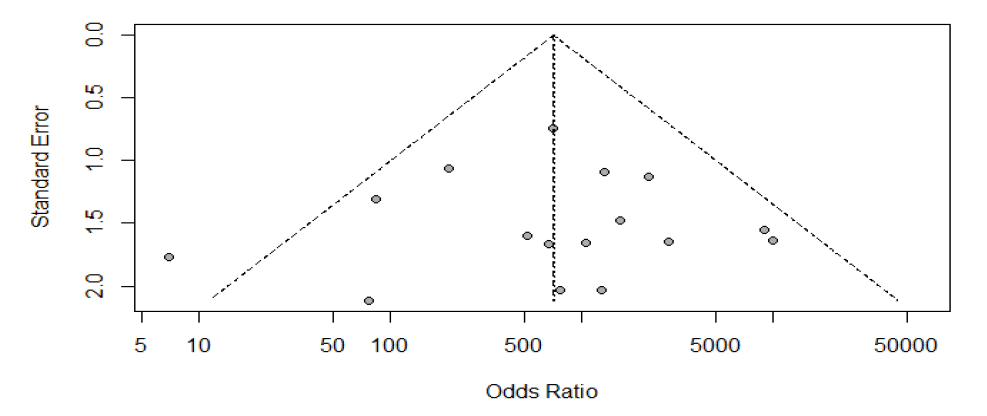
Publication bias: The summary of publication bias is shown in Table 2 and Figure 5. Out of 16 RCTs, For the publication bias, In patient selection, bias was low in 15 studies, and unclear in 1. In the index test, it was low in 16 studies. While the reference standard was low in 7, and unclear in 9. The flow and timing were low in 15, and unclear in 1. The applicability concerns in patient selection were low in 16 studies. The reference standard was low in 16. And the index test was low in 16 studies (Figure 6).
|
|
Risk of bias |
Concerns about applicability |
|||||
|---|---|---|---|---|---|---|---|
|
Reference |
Patient selection |
Index test |
Reference standard |
Flow and timing |
Patient selection |
Index test |
Reference standard |
|
Olubaniyi et al. 2014 |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Novoa et al. |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Eom et al. |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Song et al. |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
JR Buckland |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Breeze |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Jose Cura |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
K W Kesse |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Naqvi |
Low |
Low |
Unclear |
Unclear |
Low |
Low |
Low |
|
Park et al. |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Pfeiffer and Ridder |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Pratap et al. |
Unclear |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Haldar et al. |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Taki et al. |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
Wan |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Huang et al. |
Low |
Low |
Unclear |
Low |
Low |
Low |
Low |
Tab. 2. Risk of bias and applicability concern.
Fig.5. Summary of risk bias and applicability concern.
Fig.6. Funnel plot for publication bias.
Discussion
The preoperative diagnosis of salivary gland masses carries significant inefficiencies in terms of accuracy as well as diagnostic lead time, primarily due to the difficulty of diagnosing low-grade carcinomas by cellular morphology alone. However, the diagnosis of salivary tumors is important to avoid unnecessary surgery on non-tumorous conditions, as well as for treatment planning of malignant tumors in a manner that prioritizes patient well-being, considering that the performance of revision surgery in a previously operated patient generates higher costs and is related with increased technical difficulties. As not all surgical decisions can be made intraoperatively without the informed consent of our patients, a correct preoperative diagnosis is of particular importance for patient counseling and planning of the operative procedure. Moreover, cytology and histology of salivary gland neoplasms show a highly diverse mixture of growth patterns and cell types, and overlapping morphologic features, which is a diagnostic challenge even for experienced pathologists. As stated before, the aim of this study pertains to exploring and establishing two diagnostic modalities, namely Fine Needle Aspiration (FNA) and Core Needle Biopsy (CNB) for the diagnosis of salivary masses.
FNAC underlines a sensitivity of 93%, a specificity of 98.5%, and a positive predictive value of 0.972, establishing promising value as a diagnostic tool from a pool of 16 studies. It is safe, quick to perform, minimally invasive, and has been the traditional biopsy method of choice in the major salivary glands. There is however significant variability in its performance and diagnostic accuracy across various institutions, dependent upon operator experience, use of ultrasound guidance, adequacy of sample, interpretative skills, on-site presence of a cytologist, and finally, the availability of ancillary methods such as flow cytometry for additional analysis [20]. Therefore, the high specificity and sensitivity reported in some centers is not generally reproducible where the circumstances for FNAC are not optimized. FNAC also cannot reliably differentiate low-grade lymphoma from reactive nodal hyperplasia and in most cases acts as an indicator of the need for an excision or other biopsy technique. The clinical characteristics and prognosis of these tumors are quite different according to the type, stage, and grade of the tumors, which determines the extent of surgery. FNA has the limitation of possibly providing insufficient information on uncommon histology, tumor grading, and staging of salivary masses, which have already been established to have a tricky diagnostic pattern in terms of histopathology. It is also accepted that well-differentiated adenocarcinomas can be histologically indistinguishable from benign adenomas unless there is capsular infiltration to indicate malignancy, indicating the need for a modality that circumvents this inability of FNA.
Core needle biopsy provides a larger sample with preservation of tissue architecture, which permits immunohistochemical staining. This enables tumor grading, differentiation of benign and malignant lesions, and more precise diagnosis. The status of capsular invasion can also be assessed, which enables a distinction to be made between adenoid cystic carcinoma and monomorphic adenoma, an important advantage over FNA. Core needle biopsy also does not depend on the experience of the practitioner while there are several reports showing that the success of FNA is dependent on the experience of the operator. According to these articles, the experience of the cytopathologists and operators could contribute to the variability of results, and this is not often reported [6]. It is more accurate, less heterogeneous in performance, and is associated with lower non-diagnostic rates compared to FNAC. The advantages being stated rightfully, CNB imparts a particular set of demerits in its application. It is more invasive than FNAC and uses a larger bore needle. Haematoma due to minor bleeding is documented in 1-2% of cases in the parotid gland. However, there are no reports of major complications or death in this or other studies evaluating the submandibular gland. Tumor seeding is an uncommon complication with only two reported cases in the parotid gland and appears to be related to tumor type, site of puncture, and increasing needle diameter.
Conclusion
In conclusion, core needle biopsy has significant advantages over fine needle aspiration and although more invasive, promises a more accurate diagnosis. There is perhaps room for the inclusion of FNA as a primary investigation, but the risk of a missed diagnosis is bothersome, the quantification of which is beyond the scope of this study.
Limitations
Our study is not without limitations. We could perhaps gain more clarity by taking into account more studies, more subgroups of patients as well as ethnicity-centered variations.
However, to the best of our analysis, we come to the conclusion that CNB supersedes FNA as a diagnostic modality for the assessment of salivary masses.
References
- Stenner M, Klussmann JP. Current update on established and novel biomarkers in salivary gland carcinoma pathology and the molecular pathways involved. Eur Arch Otorhinolaryngol. 2009; 266:333-341.
[Crossref] [Google Scholar] [PubMed]
- Carvalho MB, Soares JM, Rapoport A, Sobrinho AJD, Fava AS, et al. Perioperative frozen section examination in parotid gland tumors. Sao Paulo Med J. 1999; 117:233-237.
[Crossref] [Google Scholar] [PubMed]
- Tan LG, Khoo ML. Accuracy of fine needle aspiration cytology and frozen section histopathology for lesions of the major salivary glands. Ann Acad Med Singap. 2006; 35:242.
[Google Scholar] [PubMed]
- Westra WH. Diagnostic difficulties in the classification and grading of salivary gland tumors. Int J Radiat Oncol. 2007; 69:S49-S51.
[Crossref] [Google Scholar] [PubMed]
- Pfeiffer J, Ridder GJ. Diagnostic value of ultrasound-guided core needle biopsy in patients with salivary gland masses. Int J Oral Maxillofac Surg. 2012; 41:437-443.
[Crossref] [Google Scholar] [PubMed]
- Eom HJ, Lee JH, Ko MS, Choi YJ, Yoon RG, et al. Comparison of fine-needle aspiration and core needle biopsy under ultrasonographic guidance for detecting malignancy and for the tissue-specific diagnosis of salivary gland tumors. Am J Neuroradiol. 2015; 36:1188-1193.
[Crossref] [Google Scholar] [PubMed]
- Kocjan G, Nayagam M, Harris M. Fine needle aspiration cytology of salivary gland lesions: advantages and pitfalls. Cytopathol. 1990; 1:269-275.
[Crossref] [Google Scholar] [PubMed]
- Raab SS, Sigman JD, Hoffman HT. The utility of parotid gland and level I and II neck fine-needle aspiration. Arch Path Lab Med. 1998; 122:823.
[Google Scholar] [PubMed]
- Stewart CJ, MacKenzie K, McGarry GW, Mowat A. Fine-needle aspiration cytology of salivary gland: A review of 341 cases. Diagn Cytopathol. 2000; 22:139-146.
[Crossref] [Google Scholar] [PubMed]
- Balakrishnan K, Castling B, McMahon J, Imrie J, Feeley KM, et al. Fine needle aspiration cytology in the management of a parotid mass: A two centre retrospective study. Surgeon. 2005; 3:67-72.
[Crossref] [Google Scholar] [PubMed]
- Brachtel EF, Pilch BZ, Khettry U, Zembowicz A, Faquin WC. Fine-needle aspiration biopsy of a cystic pleomorphic adenoma with extensive adnexa-like differentiation: Differential diagnostic pitfall with mucoepidermoid carcinoma. Diagn Cytopathol. 2003; 28:100-103.
[Crossref] [Google Scholar] [PubMed]
- Cohen EG, Patel SG, Lin O, Boyle JO, Kraus DH, et al. Fine-needle aspiration biopsy of salivary gland lesions in a selected patient population. Arch Otorhinolaryngol Head Neck Surg. 2004; 130:773-778.
[Crossref] [Google Scholar] [PubMed]
- Contucci AM, Corina L, Sergi B, Fadda G, Paludetti G. Correlation between fine needle aspiration biopsy and histologic findings in parotid masses. Personal experience. Acta Otorhinolaryngol Ital. 2003; 23:314-318.
[Google Scholar] [PubMed]
- Paris J, Facon F, Pascal T, Chrestian MA, Moulin G, et al. Preoperative diagnostic values of fine-needle cytology and MRI in parotid gland tumors. Eur Arch Otorhinolaryngol. 2005; 262:27-31.
[Crossref] [Google Scholar] [PubMed]
- Weinberger MS, Rosenberg WW, Meurer WT, Robbins KT. Fine-needle aspiration of parotid gland lesions. Head Neck. 1992; 14:483-487.
[Crossref] [Google Scholar] [PubMed]
- Hakala T, Kholová I, Sand J, Saaristo R, Kellokumpu-Lehtinen P. A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol. 2013; 66:1046-1050.
[Crossref] [Google Scholar] [PubMed]
- Samir AE, Vij A, Seale MK, Desai G, Halpern E, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid. 2012; 22:461-467.
[Crossref] [Google Scholar] [PubMed]
- Burke C, Thomas R, Inglis C, Baldwin A, Ramesar K, et al. Ultrasound-guided core biopsy in the diagnosis of lymphoma of the head and neck. A 9 year experience. Br J Radiol. 2011; 84:727-732.
[Crossref] [Google Scholar] [PubMed]
- Olubaniyi BO, Chow V, Mandalia U, Haldar S, Gok G, et al. Evaluation of biopsy methods in the diagnosis of submandibular space pathology. Int J Oral Maxillofac Surg. 2014; 43:281-285.
[Crossref] [Google Scholar] [PubMed]
- Novoa E, Gürtler N, Arnoux A, Kraft M. Diagnostic value of core needle biopsy and fine-needle aspiration in salivary gland lesions. Head Neck. 2016; 38:E346-E352.
[Crossref] [Google Scholar] [PubMed]