Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 12
Correlation between PD-L1 expression, demographic and pathological characters in patients with breast cancer
Ghasaq Mohammed Muhibul1*, Eman Tariq Ali1 and Maitham Ali Alrikabi22Department of Clinical Pharmacy, College of Pharmacy, University of Basrah, Basrah, Iraq
Ghasaq Mohammed Muhibul, Department of Clinical Laboratory Sciences, College of Pharmacy, University of Basrah, Iraq, Email: ghasaqqmohamed1991@gmail.com
Received: 26-Nov-2022, Manuscript No. OAR-22-81078; Accepted: 16-Dec-2022, Pre QC No. OAR-22-81078 (PQ); Editor assigned: 28-Nov-2022, Pre QC No. OAR-22-81078 (PQ); Reviewed: 12-Dec-2022, QC No. OAR-22-81078 (Q); Revised: 15-Dec-2022, Manuscript No. OAR-22-81078 (R); Published: 20-Dec-2022, DOI: 0
Abstract
The expression of Programmed Death-Ligand 1 (PD-L1) is associated with immune response evasion in breast cancer, making patients qualified for PD-L1 inhibitors. To better understand the prognostic value of PD-L1 in breast cancer, the aim of the study to discover the correlation between PDL1 expression and pathological characteristics and the prognostic feathers for breast cancer patients and compare the expression of PD-L1 level for both pre-treatment and post-treatment groups. In total, 142 female patients with breast cancer and 60 healthy females as control were enrolled in the study. ELISA technology was used to evaluate serum PDL-1 expression. Other patient information and tumour data were obtained from pathology reports. At a diagnosis, the mean age for total patients was (50.59 ± 12.61) years range (23-87). The higher PDL-1 expression was in (40-69) age group.PDL- 1 was positively significantly associated with age (P=0.001), family history (p=0.0001), and menopausal status (p=0.02). No significant association was found between PD-L1 expression and body mass index. The tumour stage was significantly associated with PD-L1 expression (p=0.0001); the higher expression was found in patients with advanced stages of tumour III and IV (p=0.0001). There were significant differences in the expression of PD-L1 between pre-treatment and post-treatment groups in all stages (p=0.0001). Significantly the PD-L1 expression was associated with metastasis (p=0.0001), and there were significant differences in metastasis patients than nonmetastasis for both groups. Significant associations were observed between PD-L1 expression with tumour grade, tumour size, lymph node status, and lymph vascular invasion (p=0.0001). There is a strong positive correlation between the expression of PDL-1 with age, stages, grades, tumour size, lymph node status, and metastasis. Serum expression of PD-L1 was associated to worse prognosis and progressive pathologic characteristics in breast cancer
Keywords
breast cancer, programmed cell death ligand 1 PD-L1, pathologic parameters, disease stage, metastasis
Introduction
Cancer cells are distinguished by their capacity to evade the immune response via a variety of mechanisms for escaping from tumours [1,2]. Immuno-editing protects malignant tumours from immune surveillance, which allows for the selection of immune effector-resistant tumour variants and the establishment of an immunosuppressive status within the tumour microenvironment [3]. Tumour evasion by the immune system is mediated by various mechanisms [4]. Establishing a status of immune tolerance is currently identified as a key immune evasion mechanism in cancer cells, and the employing of immune checkpoints to suppress the cell-mediated immune response [3]. Immune checkpoints are inhibitory immune receptors that principally function to maintain self-tolerance and evade immune response overstimulation [5]. Numerous immune checkpoints have been identified in cancers, but the Programmed Death-1 (PD-1) and its ligand, Programmed Death-Ligand 1 (PD-L1), are of particular importance [6]. T cell function and activation are blocked when PD-1 binds to PD-L1 [7]. Preventing the immune response and maintaining tolerance to self-antigens due to PD-1/PD-L1pathway activation under normal conditions. PD-L1 expression in tumours, however, is associated with a reduced immune response in the tumour microenvironment [8]. In several solid cancers, PD-L1 is overexpressed on tumour cells [9-13].
Breast cancer is the most common cancer in women worldwide and the second leading cause of death in cancer patients [14-16]. PD-1 and PD-L1 expression in breast cancer patients have been described in various studies and considerable differences occur in the prognostic impact and expression rates. Nevertheless, the prognostic and predictive effects of the expression of PD-L1 in breast cancer lack unanimity [17]. Atzolizumab (a PD-L1 inhibitor) and Abraxane chemotherapy were recently approved as a combination therapy for the treatment of patients with PD-L1- positive [7]. Immune checkpoint inhibitors are likely to benefit patients with other breast cancer subtypes. We need to study the prevalence expression of immune checkpoint markers in breast cancer patient populations before implementing individual treatment protocol guidelines to provide guidance on potential benefits.
Therefore, data on PD-L1 expression in Iraqi breast cancer patients is lacking. As a result, our study is the first in Iraq to address these characteristics. To better understand the prognostic value of PD-L1 in breast cancer, we conducted a present study to estimate the expression of PDL-1 in different stages of disease and metastasis and to explore the correlation between the PDL-1 expressions with pathological characteristics in breast cancer. Such a study, to our knowledge, has not been conducted, particularly in Iraq
Methods
Study design
A retrospective study was done from October 2021 to March 2022. A total of 202 adult females with breast cancer in the age range (23-87) years were enrolled in the study. Only 142 were confirmed histologically and clinically (radiologically) with breast cancer by a specialist oncologist while visiting Oncology Center in (Al-Haboubi Teaching Hospital in Al-Nasiriya city) southern Iraq. Out of the 142 breast cancer patients, 82 were enrolled before starting any treatment protocol regardless of stage and grade, known as (Pre-treatment group). The other 60 breast cancer patients were enrolled after they received different treatment protocols such as Radiotherapy and or hormonal therapy if hormone receptors positive: Tamoxifen or Letrozole, Anastrozole according to menopausal status, or if Her2 positive: Trastuzumab, and different chemotherapeutic agent as (Docetaxel, Paclitaxel, Doxorubicin, Cyclophosphamide, Gemcitabine, Carboplatin, Zoledronic acid, Palbociclib, Capecitabine, etc.). Sixty healthy females matching in age were enrolled as a control group. Before enrolling in the study, all patients and healthy controls provided written informed consent. This study was approved by both the Continuing Medical Education section in ThiQar Health Department and the Scientific Committee at the University of Basra’s College of Pharmacy.
Retrieval of demographic and anthropometric data
Both patients and healthy subjects had provided permission and consent before sample collection. The demographic and anthropometric data, including (age, body weight, family history, menopausal status, and BMI) were collected from the electronic database at the Oncology centre in Al-Haboubi Teaching hospital for breast cancer patients. The Body Mass Index (BMI) was calculated by the World Health Organization (WHO) as the patient's height in meters squared divided by their weight in kilograms. The WHO classification system was used to categorize patients as underweight, normal, overweight, or obese.
Tumour data
The Pathological data included tumour stage, tumour grade, the size and site of the tumour, histological and molecular type of breast cancer, reports generated by the pathology department of (Al-Hussain Teaching Hospital) for patients who were qualified at the time of breast cancer diagnosis were used to determine the status of ipsilateral axillary lymph nodes, lymph vascular invasion (LVI), and metastasis. The Union for International Cancer Control (Union International Centre le Cancer; UICC) determined Tumour-Node-Metastasis (TNM) staging [18]. TNM Classification of Malignant Tumours is the name for classifying malignant tumours (UICC-TNM). The Nottingham combined histologic grade system was used to determine the tumour grade.
Sample collection and serum PD-L1 measurement
3 ml venous blood specimens were collected by syringe after taking permission from both healthy subjects and patients with breast cancer during their visit to the Oncology department. Blood samples were centrifuged to separate the serum, which was then stored at -80°C for use in the determination of serum level of PD-L1 by ELISA kit (Human PD-L1 EKISA kit, Catalog No: E-EL-H1547 Elabscience, USA) having a sensitivity of (0.10 ng/ mL). Based on the manufacturer's protocol, the concentration of sPD-L1 in each sample was determined using a standard linear curve of known sPD-L1 concentrations ranging from 0.16 to 10 ng/mL evaluated in the serum of patients and control groups by using enzyme-linked immune-sorbent assay (Elisa microplate reader, Bio Tek, USA and Elisa microplate washer, Human, Germany) with commercially available kits. According to the instruction provided by the manufacturer.
Statistical analysis
The Shapiro-Wilk test was used to determine whether the data was normally distributed. For data with a normal distribution, parametric statistical tests were used; otherwise, nonparametric analyses were used. The SPSS statistical package version 24.0 (SPSS Inc., Chicago, IL, USA) was used to achieve data analysis. Continuous variables are presented as the mean ± SD; categorical variables are shown as frequency and percentages. Students' unpaired two-sided t-test and the Mann-Whitney U test were used to compare variables with and without normal distribution. While the Kruskal-Wallis one-way analysis of variance and ANOVA one-way were used to compare multiple independent groups (Pre-treatment, Post-treatment, and healthy control). Some Statistical analysis was performed using a student’s t-test with Graph Pad Prism 6 software (Graph Pad Software, Inc., La Jolla, CA, USA) for measured the expression of PD-L1 according to their (stage and metastasis) for both pre-treatment and posttreatment groups. To assess correlation between continuous variables, Pearson's correlation test was used. While to analyse correlations between categorical variables, the Spearman rank correlation (rho) test was used (Nonparametric) Correlations. At p<0.05, all p-values were statistically significant.
Results
Patients' demographic and pathological characteristics are illustrated in (Table 1). Age distribution indicated a significant difference between the pre-treatment and post-treatment groups (p=0.03), and a higher proportion of the participants belonged to the 40 years’ -69 years’ age group. There is no discernible difference in BMI levels seen between the groups. The majority of the patients in the pre-treatment group were (40.2%) obese, while in the post-treatment group majority were (55%) overweight. No significant difference in a first-degree relative diagnosed with breast cancer was seen between the groups. Our data confirm that most of the patients in both groups had a negative family history of breast cancer (90.8 %), as shown in table 1. There was a significant difference (p=0.02) between the pre-treatment and post-treatment groups in menopause status. A higher proportion in post-menopausal was (65%) compared to the pre-treatment group (41.5%). The distribution of tumour site and tumour size were not significantly different between the two groups (p=0.05 and p=0.08, respectively). No statistically significant difference in the distribution of lymph node status, disease stage, tumour grade, LVI, and metastasis between groups.
Tab. 1. Demographic and pathological characteristics in breast cancer patients.
| Characteristic | Total patients (N=142) in percentage | Pre-treatment (N=82) in percentage | Post-treatment (N=60) in percentage | P≤* |
|---|---|---|---|---|
| Age (year) | ||||
| 20-39 | 33 (23.2) | 24 (29.3) | 9 (15) | 0.03 |
| 40-69 | 94 (66.2) | 46 (56.1) | 48 (80) | |
| ≥ 70 | 15 (10.6) | 12 (14.6) | 3 (5) | |
| Mean ± SD | 50.59 ± 12.61 | 49.97 ± 14.79 | 51.45 ± 9.23 | 0.1 |
| Range | 23-87 | (23-87) | (36-70) | |
| BMI | ||||
| Underweight | 0 | 0 | 0 | 0.13 |
| Normal | 24 (16.9) | 18 (22) | 6 (10) | |
| Overweight | 64 (44.4) | 31 (37.8) | 33 (55) | |
| Obese | 54 (38.7) | 33 (40.2) | 21 (35) | |
| Family history | ||||
| Positive | 13 (9.2) | 10 (12.2) | 3 (5) | 0.33 |
| Negative | 129 (90.8) | 72 (87.8) | 57 (95) | |
| Type of patients | ||||
| Pre-treatment | 82 (57.7) | 82 (100) | 0 | |
| Post-treatment | 60 (42.3) | 0 | 60 (100) | |
| Menopausal state | ||||
| Premenopausal | 69 (48.6) | 48 (58.5) | 21 (35) | 0.02 |
| Post menopause | 73 (51.4) | 34 (41.5) | 39 (65) | |
| Tumour Site | ||||
| Left | 80 (56.3) | 38 (46.3) | 42 (70) | 0.05 |
| Right | 53 (37.3) | 38 (46.3) | 15 (25) | |
| Bilateral | 9 (6.3) | 6 (7.3) | 3 (5) | |
| Tumour Size | ||||
| T0 | 2 (1.4) | 2 (2.4) | 0 | 0.08 |
| Tis | 2 (1.4) | 2 (2.4) | 0 | |
| T1 | 25 (27.6) | 10 (12.2) | 15 (25) | |
| T2 | 60 (42.3) | 42 (48.8) | 18 (30) | |
| T3 | 32 (22.5) | 14 (19.5) | 18 (30) | |
| T4 | 21 (14.8) | 12 (14.6) | 9 (15) | |
| Lymph node status | ||||
| N0 | 43 (30.3) | 28 (34.1) | 15 (25) | 0.27 |
| N1 | 39 (27.5) | 18 (22) | 21 (35) | |
| N2 | 31 (21.8) | 16 (19.5) | 15 (25) | |
| N3 | 29 (20.4) | 20 (24.4) | 9 (15) | |
| Disease stage | ||||
| 0 | 2 (1.4) | 2 (2.4) | 0 | 0.47 |
| â? | 24 (16.9) | 12 (14.6) | 12 (20) | |
| â?¡ | 41 (28.9) | 26 (31.7) | 15 (25) | |
| The | 41 (28.9) | 26 (31.7) | 15 (25) | |
| â?£ | 34 (23.9) | 16 (19.5) | 18 (30) | |
| Tumour grade | ||||
| â? | 18 (12.7) | 12 (14.6) | 6 (10) | 0.71 |
| â?¡ | 45 (31.7) | 24 (29.3) | 21 (35) | |
| â?¢ | 79 (55.6) | 46 (56.1) | 33 (55) | |
| LVI | ||||
| Present | 86 (60.6) | 50 (61) | 36 (60) | 0.918 |
| not seen | 56 (39.4) | 32 (39) | 24 (40) | |
| Metastasis | ||||
| M0 | 108 (76.1) | 66 (80.5) | 42 (70) | 0.25 |
| M1 | 34 (23.9) | 16 (19.5) | 18 (30) | |
Age character was significantly associated with PD-L1 expression in the pre-treatment group. (p=0.001). The patients in the age group (≥ 70 years) had lower expression of PDL-1 level (5.51 ng/ml ± 2.37 ng/ml) as compared to other age groups. However, patients aged 40 to 69 years had the highest expression of PD-L1 levels (6.52 ng/ml ± 2.18 ng/ml) compared to other age groups. Furthermore, there was no significant association between PDL1 level and BMI. In contrast, a significant association was found between family history and PD-L1 expression (P<0.0001). Patients with a familial risk factor had significantly lower expression of PD-L1 level (3.43 ng/ml ± 1.12 ng/ml) than those without a family history (6.13 ng/ml ± 2.14 ng/ml). A significant association was observed between menopause and the expression of PDL-1 (p=0.02). Cases with premenopausal had significantly lower expression of PDL-1 levels as compared to post-menopausal patients (5.33 ng/ml ± 2.08 ng/ml, 647 ng/ml ± 2.28 ng/ml), Table 2.
Tab 2. Association expression of PDL-1 with demographic characters in the pre-treatment group.
| Parameters | PD-L1 | ||
|---|---|---|---|
| Age groups | Mean | SD | P ≤ * |
| 20-39 | 4.57 | 1.69 | 0.001 |
| 40-69 | 6.52 | 2.18 | |
| ≥ 70 | 5.51 | 2.37 | |
| BMI | |||
| Underweight | 0 | 0 | 0.25 |
| Normal | 5.84 | 2.43 | |
| Overweight | 5.31 | 1.96 | |
| Obese | 6.24 | 2.32 | |
| Family History | |||
| Positive | 3.43 | 1.12 | |
| Negative | 6.13 | 2.14 | 0.0001 |
| Menopausal status | |||
| Premenopausal | 5.33 | 2.08 | 0.02 |
| Postmenopausal | 6.47 | 2.28 | |
There was a significant association between PD-L1 expression and tumour grade (p<0.0001). Patients with higher grade (III) had higher expression of PDL-1(6.58 ng/ml ± 2.12 ng/ml) than other grades (Table 3). Additionally, our findings demonstrated a statistically significant association between tumour size and PD-L1 expression (p 0.0001). High expression of PD-L1 was observed in the patients with larger tumour size T3 (7.87 ng/ml ± 1.68 ng/ml). PD-L1 expression was also significantly associated with lymph node status (p<0.0001). High-level expression of PDL1 was observed in the patients with higher involvement of lymph nodes N3 (7.81 ng/ml ± 1.64 ng/ml) than other N0, N1, and N2 (3.87 ng/ml ± 1.14, 5.32 ± 1.79, 7.21 ± 1.63 ng/ml). Moreover, a significant association was observed between LVI and PD-L1 levels (p<0.0001). Patients with LVI had significantly higher levels of PD-L1 than those without LVI, as shown in Table 3.
Tab 3. Association expression of PD-L1 with pathological characteristics in pre-treatment group.
| Characteristics | PD-L1 | ||
|---|---|---|---|
| Tumour grade | Mean | SD | P ≤ * |
| I | 3.43 | 1.13 | 0.0001 |
| II | 5.51 | 1.96 | |
| III | 6.58 | 2.12 | |
| Tumour size | |||
| T0 | 4.48 | 1.94 | 0.0001 |
| Tis | 2.43 | 1.54 | |
| T1 | 2.89 | 0.36 | |
| T2 | 5.41 | 1.65 | |
| T3 | 7.87 | 1.68 | |
| T4 | 7.57 | 1.45 | |
| Lymph Node Status | |||
| N0 | 3.87 | 1.14 | 0.0001 |
| N1 | 5.32 | 1.79 | |
| N2 | 7.21 | 1.63 | |
| N3 | 7.81 | 1.64 | |
| LVI | |||
| Present | 7.03 | 1.84 | 0.0001 |
| Not seen | 3.89 | 1.18 | |
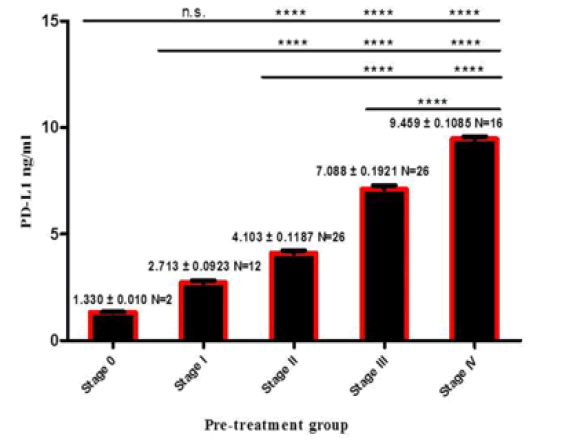
A significant association was observed between PD-L1 expression and disease stages (p<0.0001) figure 2. The PD-L1 expression was significantly higher in patients with advanced tumour stages (stage III and IV) (7.286 ng/ml ± 0.2265 ng/ml, 9.459 ng/ml ± 0.1085 ng/ml), respectively (Figure 1).
Figure 1: . The PD-L1 levels in different tumour stages.
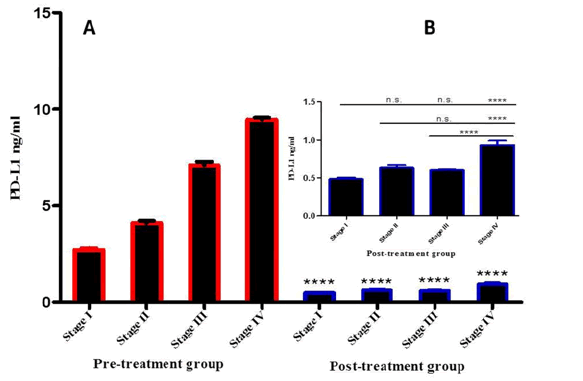
There was a significant difference in PD-L1 expression between the two groups in all disease stages (p<0.0001). Also level of PDL1 was higher in stage IV than other stages in post treatment group (p<0.0001) (Figure 2).
Figure 2: PD-L1 expression was compared at different stages in both pre- and posttreatment groups.
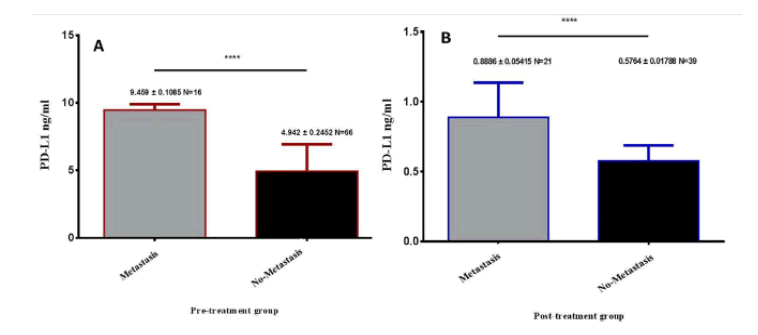
There was a significant difference in the expression of PD-L1 level in metastasis patients than in non-metastasis cases (p <0.0001). The level expression of PDL-1 in metastasis was higher than nonmetastasis (9.459 ng/ml ± 1.085 ng/ml; 4.942 ng/ml ± 0.245 ng/ ml) (Figure 3A).There was a significant difference in the expression of PD-L1 level in metastasis cases than non-metastasis cases in the post-treatment group (P<0.0001). The mean level of PD-L1was 0.8886 ng/ml ± 0.5415 ng/ml higher significance (Figure 3B).
Figure 3: Expression of PD-L1 level in metastasis and non-metastasis patients. (A) PD-L1 level in metastasis and non-metastasis cases pre-treatment breast cancer patients. (B) PD-L1 level in metastasis and non-metastasis cases post-treatment breast cancer patients.
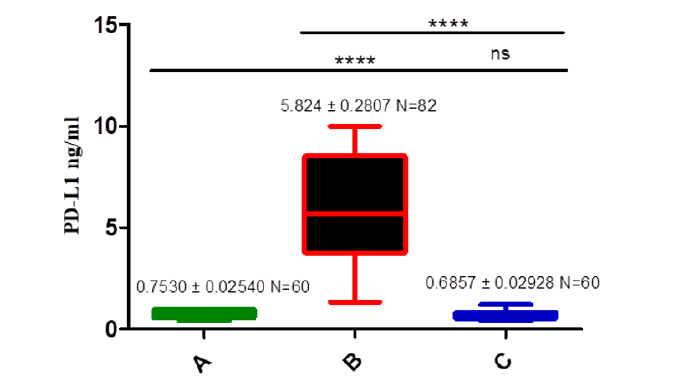
The mean level of PD-L1 in the pre-treatment group was significantly higher than in the post-treatment and control groups (5.824 ng/ml ± 0.2807 ng/ml, 0.7642 ng/ml ± 0.04340 ng/ ml, 0.7530 ng/ml ± 0.02540 ng/mL) respectively, (p<0.0001). Inversely there is no significant difference between healthy control and post-treatment groups, as shown in Figure 4.
Figure 4: Comparison serum level of PD-L1 in different groups. A is a Control group, B is a Pre-treatment group, C is a Post treatment group.
Our results confirmed that the expression of PD-L1 level was positively correlated with age, family history, and menopausal state (r=0.230*, p=0.03; r=0.405, p=0.0001; r=0.243, p=0.02) respectively. However, there was not a significant correlation reported between PD-L1 expression and either BW or BMI, as indicated in the results (Table 4).
Tab. 4. Correlation between PD-L1 expression and demographic in pre-treatment patients group.
| Age | BW | BMI | Family history | Menopausal State | ||
|---|---|---|---|---|---|---|
| PD-L1 | r | 0.234* | -0.029 | -0.077 | 0.405 | 0.243 |
| Sig. (2-tailed) | 0.03* | 0.794 | 0.489 | 0.0001 | 0.028 |
As Table 5 shows, there was a significant strong positive correlation between PDL-1 expression and clinical pathological characters stage, grade, tumour size, LNS, and metastasis (rho=0.880**, P=0.0001; rho=0.470, P=0.0001; rho=0.730**, P=0.0001;0.730, P=0.0001; rho=0.620, P=0.0001) respectively.
Tab 5. The correlation between PD-L1 expression and pathological characteristics in the pretreatment group
| Pathological characters | PD-L1 | |
|---|---|---|
| **rho | P | |
| Tumour Stage | 0.756** | 0.0001 |
| Tumour Grade | 0.466** | 0.0001 |
| Tumour size | 0.598** | 0.0001 |
| Lymph Node Status | 0.735** | 0.0001 |
| Metastasis | 0.623** | 0.0001 |
Discussions
The most prevalent cancer among women in Iraq is breast cancer, and the incidence of the disease is increasing yearly [19]. Inhibitors of immune checkpoints were recently licensed for the treatment of a variety of solid cancers. Moreover, their indicators continue to rise. Understanding the expression landscape of target immune checkpoints is therefore essential for delineating the status of anti-tumour immunity and further investigating the eligibility of breast cancer patients in Iraq for immunotherapy. The fact that Iraqi breast cancer patients have some different demographic characteristics than Western populations adds. These demographic characteristics are well represented in our findings. They include younger mean age at diagnosis (50.59 ± 12.61) years range (23- 87), (9.2%) of positive breast cancer family history in first-degree relatives and the prevalence of premenopausal breast cancer cases is high (48.6%).
Through our results, this fact is confirmed that the demographic and clinic-pathological factors which were found to be significantly associated with the PD-L1 expression were: age, family history, BMI, and menopausal state but the most factor associated with the positive expression of PD-L1 was age, and the highest expression of PD-L1 in women with age group range (40-69 years), This result is consistent with the findings of a previous study [20], maybe this due to the immune-senescence as a plausible explanation for this finding, As well as the period of hormonal change, menopause, and the quality of life of this population was considered one of the reasons within this age group (40-69). Many studies confirm these findings; Alice M Alves et al. reported that the average age of patients with positive PD-L1 expression was (61 ± 11.36) years [21]. Additionally, Chenxi Yuan et al. reported that the PD-L1 expression is significantly higher in patients older than 50 years [22]. Moreover, the current study confirmed that the expression of PD-L1 in a patient’s serum is positively correlated with age. On the other hand, we did not find any correlation between PD-L1 expression and BMI, Although the highest PD-L1 expression was in obese patients. This finding is in contract with the previous study, which was similar to our findings [23].
As for family history, Patients with a positive breast cancer family history (9.2%) had significantly lower expression of PD-L1 than those without a family history (90.8%). This could be explained by the small size of patients enrolled in the study (142). This is in contract to Baglia et al. reported that first-degree family history of breast cancer might be an important risk factor for the development of second primary breast cancer among women with a previous in situ breast cancer and patients with a positive family history of breast cancer has significantly raised PD-L1 [24]. At the same time, premenopausal women with breast cancer (48.6%) had significantly lower expression of PD-L1 as compared to postmenopausal (51.4%) [25].
In the same scenario, the present study demonstrated a significant association between PD-L1 expression and pathological feathers of breast cancer women (family history, lymph vascular invasion,tumour stage, tumour grade and size, lymph node status, and metastasis). In addition, the results illustrated that PD-L1 expression had a significant positive correlation with pathological features of breast cancer, including (Disease stage, tumour grade, size, lymph node status, and metastasis). These results are consistent with a number of earlier studies that have demonstrated the correlation between PD-L1 and unfavourable pathological characteristics, including an advanced grade, evidence of lymph nodes that are positive for cancer, negative hormone receptors, and larger tumour size [20,26,27]. Huang et al. examined 47 studies and found that PD-L1 positive was related to high-grade tumours and large tumour size [26]. PD-L1 expression was substantially correlated with patient age, tumour size and grade, and lymph node status, as demonstrated by Muenst et al. [28]. Chenxi Yuan et al. reported a statistically significant difference in PD-L1 expression observed between the primary tumours and paired metastatic lymph nodes [21,22]. Li et al. discovered in a recent study that PD-L1 expression was stronger and more frequent in lymph node metastases than in paired primary breast tumours [29]. This can be justifiable as the expression of PD-L1 in metastatic carcinoma may be due to the dynamic nature of PD-L1, which changes with tumour progression [29,30].
A significant association was observed between Lymph vascular invasion and PD-L1 levels. G Houvenaeghel et al. studied the Lymph Vascular Invasion(LVI) as a significant prognostic impact in patients with early breast cancer, results from a large cohort and reported that the presence of LVI was significantly associated with a negative prognostic impact on overall, diseasefree, and metastasis-free survival (MFS) in all patients and the elevated tumour markers were also observed [31]. Few studies have also reported the significant association of PD-L1 with other carcinomas. Mitchell et al. assessed the LVI association with PD-L1 in resected lung carcinoma and found on multivariable analysis LVI was associated with synchronous elevated PD-L1 expression [32].
Several hypotheses can explain this pattern of expression. First, according to several studies, interferons (IFNs), which are produced by infiltrating immune cells, induce an increase in PD-L1 expression in tumour cells [33]. Second, the absence of phosphatase and tensin homolog PTEN expression, which is a regulatory mechanism for PD-L1 expression in triple-negative breast cancer TNBC patients [34], as previously described in patients with glioma [35]. The discrepancy in PD-L1 expression between primary tumour and lymph node metastasis may also be enhanced by clonal selection [36]. Additionally, according to one study, basal-like breast cancer cells can evade the immune system by regulating the PD-1 ligand adaptive IFN-c, which is secreted by helper T cells [37]. Therefore, during the infiltration of tumour cells, enriched T cells may promote the expression of PD-L1 to cause adaptive immune resistance [38].
Our finding revealed that the PD-L1 expression significantly varied between the pre-treatment and post-treatment groups when compared to the control. Our finding revealed that PD-L1 expression was significantly higher in the pre-treatment group as compared to the post-treatment group or control group, and this agrees with the previous study [25]. Several study confirmed that chemotherapeutics drugs can enhanced PD-L1 expression in tumour tissues [39]. PD-L1 expression was shown to be higher in breast cancer patients who were from the Middle East and Brazil [13,40]. On the contrary, another study by Ayoub et al. found that Jordanian female patients diagnosed with breast cancer, the expression of PD-L1 level is relatively low frequency [41]. Among the plausible explanations for these findings is that the rates of PD-L1 expression can vary significantly because there is currently no standardized method for scoring. Additionally, Differences between the cut-off values for PD-L1 positive in clinical research and the number of primary antibodies available for staining [42].
Other possible causes for the discrepancies can be clarified by the variability in populations of patients who had been examined and their variability in demographic and clinical features. Scientific facts confirm that under normal conditions, the immune system performs a series of steps leading to an anticancer immune response and cancer cell death, known as the cancer immunity cycle [43]. In response to endogenous immune anti-tumour activity, tumour cells use the PD-1/PD-L1 pathway as an adaptive immune resistance mechanism [43,44]. In the tumour microenvironment, PD-L1 is overexpressed on tumour cells or non-transformed cells. Inhibition of the cytotoxic T cells occurs when PD-L1 expressed on tumour cells binds to PD-1 receptors on activated T cells. 1 In the tumour microenvironment, these deactivated T-cells remain inhibited [43,44]. So, when immunotherapy is utilized against PD-1 and PD-L1, this mechanism of PD-1 and PD-L1 is blocked. Hence, the levels of these markers may significantly vary in post-treated versus pre-treated groups [25]. Assessment of the correlation of PD-L1 expression with pathological characteristics in the pre-treatment group, the post-treatment group, had low PD-L1 expression because of treatment and we measured PDL1 expression in serum not tumour cells. It cannot correlate pathological reports determined at the time of diagnosis or mastectomy with serum PD-L1 expression at the time of study when the patient started with treatment protocol for different and long periods.
The key strength of this study is that we had planned and investigated a well-characterized diagnosis set of samples. We also added a validation group (control group) for this study This helped us to explore the differential diagnostic effectiveness of immune check-point marker in a group of women placed at different levels of management. Also, it enabled us to assess the participants (post-treatment group) for the early detection and prognostication of breast cancer.
Limitations
A small number of patients with breast cancer enrolled in the present study with a short collection period. Different treatment protocols in the post-treatment group include (Radiotherapy and hormonal therapy).
Conclusion
There was a significant positive correlation between PD-L1 expressions and unfavourable pathological characteristics in breast cancer patients. PDL-1 is specific marker for prognosis breast cancer.
Conflicts of Interest
None.
Funding Statement
None.
Acknowledgement
Many thanks to Dr. Adel Auda Al-Aumairi, Specialist Oncologist at the Oncology Center of Al-Haboubi Teaching hospital in Al-Nasiriya city, Dr. Bassim Abdulrazaq Humood, General Practitioner in Oncology at the Oncology Center of Al-Haboubi Teaching hospital in Al-Nasiriya city and Dr. Hameed Naeem Musa, Histology and cytology specialist at the pathology department of Al-Hussain Teaching hospital.
References
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. InSeminars cancer biol. 2015; 35: 185-S198.[Google Scholar] [CrossRef]
- B. Journal, O. Surgery, Basrah Journal Of Surgery, 2007; 59-62.
- Ayoub NM, Al-Shami KM, Yaghan RJ. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer: Targets Ther. 2019;11:53.[Google Scholar] [CrossRef]
- Jamel ZF, Mehdi DS, Alsaimary IE, Abood RA. Clinical Investigation and Cancer Grades among Patients with Breast Cancer in Basrah City-Iraq. Clin Med Health Res J. 2021;1:52-56.[Google Scholar][CrossRef]
- He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669.[Google Scholar] [CrossRef]
- De Keukeleire SJ, Vermassen T, Hilgert E, Creytens D, Ferdinande L, et al. Immuno-oncological biomarkers for squamous cell cancer of the head and neck: current state of the art and future perspectives. Cancers. 2021;13:1714.[Google Scholar] [CrossRef]
- Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumour immune response? Front Immunol. 2017;8:1597.[Google Scholar] [CrossRef]
- Setordzi P, Chang X, Liu Z, Wu Y, Zuo D. The recent advances of PD-1 and PD-L1 checkpoint signaling inhibition for breast cancer immunotherapy. Eur J Pharmacol. 2021;895:173867.[Google Scholar] [CrossRef]
- Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11:964-975.[Google Scholar] [CrossRef]
- Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25:2178-2184.[Google Scholar] [CrossRef]
- Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncol. 2015;18:195-205.[Google Scholar] [CrossRef]
- Li Y, Liang L, Dai W, Cai G, Xu Y, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumour infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:1-5.[Google Scholar] [CrossRef]
- Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78-84.[Google Scholar] [CrossRef]
- Habib OS, Hameed LA, Ajeel NA, Al-Hawaz MH, Al-Faddagh ZA, et al. Epidemiology of breast cancer among females in Basrah. Asian Pac J Cancer Prev. 2016;17:191-195.[Google Scholar] [CrossRef]
- Al-Hawwaz MH, Abdulsamad HH, Mahmoud RA. Breast cancer among women in basrah, iraq: a descriptive study in birad 1 & 2 screened cases. Basrah J Surg. 2021;27:51-58.[Google Scholar] [CrossRef]
- Khalaf EA, Abduljaleel SA, Al-Jassani HM. Appraisal of Trace Elements and Heavy Metals Levels in Breast Cancer Patients of Basrah Province. Toxicol Int. 2021;28:8-14.[Google Scholar] [CrossRef]
- Matikas A, Zerdes I, Lovrot J, Richard F, Sotiriou C, et al. Prognostic implications of PD-L1 expression in breast cancer at the protein and mRNA levels.[Google Scholar] [CrossRef]
- Teichgraeber DC, Guirguis MS, Whitman GJ. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. Am J Roentgenol. 2021;217:278-290.[Google Scholar] [CrossRef]
- Al-Muskakeh MK, Yaseen AN, Aldabagh MA. Assessment of Soluble PD-1 and PD-L1 in Iraqi Women Patients with Breast Cancer with Toxoplasmosis. Indian J Forensic Med Toxicol. 2022;16.
[Google Scholar] [CrossRef] - Ayoub NM, Fares M, Marji R, Al Bashir SM, Yaghan RJ. Programmed Death-Ligand 1 Expression in Breast Cancer Patients: Clinicopathological Associations from a Single-Institution Study. Breast Cancer: Targets Ther. 2021;13:603.[Google Scholar] [CrossRef]
- Alves AM, Paredes J, Schmitt F. Expression of PD-L1 in primary breast carcinoma and lymph node metastases. Surgical and Experimental Pathology. 2019;2:1-6.[Google Scholar] [CrossRef]
- Gol S, Pena RN, Rothschild MF, Tor M, Estany J. A polymorphism in the fatty acid desaturase-2 gene is associated with the arachidonic acid metabolism in pigs. Sci Rep. 2018;8:1-9.[Google Scholar] [CrossRef]
- Tateishi A, Horinouchi H, Yoshida T, Masuda K, Jo H, et al. Correlation between body mass index and efficacy of anti-PD-1 inhibitor in patients with non-small cell lung cancer. Respiratory Investigation. 2022;60:234-240.[Google Scholar] [CrossRef]
- Baglia ML, Tang MT, Malone KE, Porter P, Li CI. Family History and Risk of Second Primary Breast Cancer after in Situ Breast CarcinomaFamily History and Second Primary Breast Cancer. Cancer Epidemiol Biomark Prev.2018;27:315-320.[Google Scholar] [CrossRef]
- Barclay J, Creswell J, León J. Cancer immunotherapy and the PD-1/PD-L1 checkpoint pathway. Arch Esp Urol. 2018;71:393-399.[Google Scholar]
- Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast cancer research and treatment. 2019;178:17-33.[Google Scholar] [CrossRef]
- Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, et al. PD-L1 expression and intratumour al heterogeneity across breast cancer subtypes and stages. Am J Surg Pathol. 2017;41:334-342.[Google Scholar] [CrossRef]
- Muenst S, Schaerli AR, Gao F, Däster S, Trella E, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15-24.[Google Scholar] [CrossRef]
- Li M, Li A, Zhou S, Xu Y, Xiao Y, et al. Heterogeneity of PD-L1 expression in primary tumour s and paired lymph node metastases of triple negative breast cancer. BMC cancer. 2018;18:1-9.
[Google Scholar] [CrossRef] - Wei XL, Luo X, Sheng H, Wang Y, Chen DL, et al. PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumour in colorectal cancer. J Transl Med. 2020;18:1-0.[Google Scholar] [CrossRef]
- Houvenaeghel G, de Nonneville A, Cohen M, Classe JM, Reyal F, et al. Isolated ipsilateral local recurrence of breast cancer: predictive factors and prognostic impact. Breast Cancer Res Treat. 2019;173:111-22.[Google Scholar] [CrossRef]
- Mitchell KG, Negrao MV, Parra ER, Li J, Zhang J, et al. Lymphovascular invasion is associated with mutational burden and PD-L1 in resected lung cancer. Ann Thorac Surg. 2020;109:358-366.[Google Scholar] [CrossRef]
- Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, et al. The presence of programmed death 1 (PD-1)-positive tumour -infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667-676.[Google Scholar] [CrossRef]
- Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370.[Google Scholar] [CrossRef]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, et al. Loss of tumour suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84-88.[Google Scholar] [CrossRef]
- Kurbasic E, Sjostrom M, Krogh M, Folkesson E, Grabau D, et al. Changes in glycoprotein expression between primary breast tumour and synchronous lymph node metastases or asynchronous distant metastases. Clin Proteom. 2015;12:1-4.[Google Scholar] [CrossRef]
- Karasar P, Esendagli G. T helper responses are maintained by basal-like breast cancer cells and confer to immune modulation via upregulation of PD-1 ligands. Breast Cancer Res Treat. 2014;145:605-614.[Google Scholar] [CrossRef]
- Kakavand H, Vilain RE, Wilmott JS, Burke H, Yearley JH, et al. Tumour PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28:1535-1544.[Google Scholar] [CrossRef]
- Tian Z, Yao W. PD-1/L1 inhibitor plus chemotherapy in the treatment of sarcomas. Front. Immunol. 2022:4926.[Google Scholar] [CrossRef]
- Parvathareddy SK, Siraj AK, Ahmed SO, Ghazwani LO, Aldughaither SM, et al. PD-L1 protein expression in middle eastern breast cancer predicts favorable outcome in triple-negative breast cancer. Cells. 2021;10:229.[Google Scholar] [CrossRef]
- Evangelou Z, Papoudou-Bai A, Karpathiou G, Kourea H, Kamina S, et al. PD-L1 expression and tumour -infiltrating lymphocytes in breast cancer: clinicopathological analysis in women younger than 40 years old. In Vivo. 2020;34:639-647.[Google Scholar] [CrossRef]
- Barrett MT, Lenkiewicz E, Malasi S, Basu A, Yearley JH, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20:1-5.[Google Scholar] [CrossRef]
- Wang Y, Yin Q, Yu Q, Zhang J, Liu Z, et al. A retrospective study of breast cancer subtypes: the risk of relapse and the relations with treatments. Breast Cancer Res Treat. 2011;130:489-498.[Google Scholar] [CrossRef]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10.[Google Scholar] [CrossRef]