Research - Onkologia i Radioterapia ( 2022) Volume 16, Issue 2
2Department of Radiation Oncology, JN Medical College and Hospital, Aligarh Muslim University, Uttar Pradesh, India
3Department of Obstetrics and Gynecology, JN Medical College and Hospital, Aligarh Muslim University, Uttar Pradesh, India
Vijayeta Ray, Department of Radiation Oncology, Acharya Harihar Postgraduate Institute of Cancer, Cuttack, India,
Received: 24-Jan-2022 Accepted: 10-Feb-2022 Published: 20-Feb-2022
Abstract
Most patients with epithelial ovarian cancer have the advanced-stage disease when the diagnosis is established. The survival rates of patients with advanced ovarian cancer have steadily improved as a result of both a more skilled surgical approach and the development of more effective chemotherapy in first-line treatment. Paclitaxel is an important agent in the management of patients with advanced ovarian cancer. Following cytoreductive surgery, the current optimal chemotherapeutic approach consists of a platinum compound together with paclitaxel. This recommendation is supported by level 1 evidence from two large randomized trials, the Gynecologic Oncology Group (GOG) study and the Canadian European Intergroup study that showed the paclitaxel/cisplatin combination to be superior to the cyclophosphamide/ cisplatin combination in terms of survival. Long term follow-up data from the GOG study showed the survival advantage was still maintained at 5 years in patients who received the paclitaxel/cisplatin combination compared with those who received cyclophosphamide/cisplatin (27 vs 16%). However, neurotoxicity was a significant problem, particularly in the Intergroup study, which used a higher paclitaxel dose (175-200 mg/m2 over 3 h) than the GOG study (135 mg/m2 over 24 h). One of a number of approaches to reduce toxicity has included the weekly administration of paclitaxel. The lower doses and shorter infusion times used with weekly dosing appear to minimize bone marrow suppression and other toxicities associated with standard paclitaxel 3-weekly administration. The dose-dense approach of weekly paclitaxel may also achieve greater efficacy than standard doses every 3 weeks, through more sustained exposure of dividing tumor cells to paclitaxel’s cytotoxic and anti-angiogenic effects. Attempts to improve the outcome of chemotherapy in women with advanced ovarian cancer have focused on attenuation of side-effects, improvements in responsiveness, progressive palliation of the disease and improvements in quality of life. The recommended treatment strategy for patients with advanced ovarian cancer is upfront radical cytoreductive surgery followed by combination chemotherapy with a taxane and a platinum compound . In order to increase efficacy further, three-drug regimens have also been used.
Keywords
required dose-dense taxanes, neoadjuvant therapy, ovarian cancer, advanced stage dose-dense, radiotherapy, carcinoma ovary, ovarian neoplasm, taxanes, platinum compounds, neoadjuvant chemotherapy, chemotherapy, paclitaxel, carboplatin, feasibility
Introduction
Most patients with epithelial ovarian cancer have advanced-stage disease when the diagnosis is established. The survival rates of patients with advanced ovarian cancer have steadily improved as a result of both a more skilled surgical approach and the development of more effective chemotherapy in first-line treatment. Paclitaxel is an important agent in the management of patients with advanced ovarian cancer.
Following cytoreductive surgery, the current optimal chemotherapeutic approach consists of a platinum compound together with paclitaxel [1-3]. This recommendation is supported by level 1 evidence from two large randomized trials, the Gynecologic Oncology Group (GOG) study and the Canadian European Intergroup study that showed the paclitaxel/cisplatin combination to be superior to the cyclophosphamide/cisplatin combination in terms of survival [4-6]. Long term follow-up data from the GOG study showed the survival advantage was still maintained at 5 years in patients who received the paclitaxel/cisplatin combination compared with those who received cyclophosphamide/cisplatin (27% vs 16%) [5]. However, neurotoxicity was a significant problem, particularly in the Intergroup study [6], which used a higher paclitaxel dose (175mg/m2 -200 mg/m2 over 3 h) than the GOG study (135 mg/m2 over 24 h) [4]. One of a number of approaches to reduce toxicity has included weekly administration of paclitaxel. The lower doses and shorter infusion times used with weekly dosing appear to minimize bone marrow suppression and other toxicities associated with standard paclitaxel 3-weekly administration. The dose-dense approach of weekly paclitaxel may also achieve greater efficacy than standard doses every 3 weeks, through more sustained exposure of dividing tumor cells to paclitaxel’s cytotoxic and anti-angiogenic effects [7-10].
Attempts to improve the outcome of chemotherapy in women with advanced ovarian cancer have focused on attenuation of side-effects, improvements in responsiveness, progressive palliation of the disease and improvements in quality of life.
The recommended treatment strategy for patients with advanced ovarian cancer is upfront radical cytoreductive surgery followed by combination chemotherapy with a taxane and a platinum compound. In order to increase efficacy further, three-drug regimens have also been used.
Paclitaxel plus carboplatin as neoadjuvant chemotherapy
The role of neoadjuvant chemotherapy in advanced ovarian cancer has been explored in various trials. In a retrospective analysis of the EORTC/NCIC trial, patients with stage IVB disease and bulky tumors had better 5-year survival rates with neoadjuvant therapy, whereas those with stage IIIC and less bulky tumors had a greater survival benefit with upfront surgery [11]. NCCN guidelines state that neoadjuvant chemotherapy may be considered for patients with bulky stage III to IV disease who are not surgical candidates following assessment by a gynaecologic oncologist [12]. GOG 111 was a randomized study comparing cisplatin and paclitaxel with cisplatin and cyclophosphamide in women with suboptimally debulked, large-volume ovarian cancer. The paclitaxel-containing arm demonstrated improved clinical response rates (73% vs. 60%), progression-free survival (18 vs. 13 months), and overall survival (38 vs. 24 months) [5]. A second GOG study of 614 women with advanced disease and suboptimal resection compared single-agent cisplatin to 24-hour infusion of paclitaxel and to the combination of paclitaxel and cisplatin. Cisplatin alone or in combination with paclitaxel resulted in improved clinical response rates and progression-free survival, although overall survival was similar in the three arms [18]. Combination chemotherapy also had lower cumulative toxicity. Although the GOG study showed that the combination of paclitaxel and cisplatin was more effective than cyclophosphamide/cisplatin regimens in women with advanced ovarian cancer, because cisplatin is associated with a high incidence of renal dysfunction and neurotoxic events [5,13] carboplatin has been used as a substitute due to its lower non-hematologic toxicity and similar efficacy to cisplatin [14-16].
As several trials have demonstrated the comparable efficacy of cisplatin and carboplatin, combination of carboplatin and paclitaxel has become the preferred first-line chemotherapy regimen. Although the standard dosing of intravenous carboplatin and paclitaxel is every 21 days, landmark phase III trial from Japan (JGOG 3016) demonstrated significant gains in progression-free and overall survival with a dose-dense regimen of weekly paclitaxel in combination with carboplatin given every 3 weeks [17]. With this background we are conducting this study with a purpose to compare the efficacy, tolerance and toxicities of dose dense therapy with respect to conventional 3 weekly regimen.
Aims and objectives
We aimed at comparatively finding out efficacy, tolerance and adverse reactions of dose dense weekly paclitaxel and carboplatin versus conventional 3 weekly paclitaxel and carboplatin in neoadjuvant treatment of ovarian cancer in terms of:
• Response to therapy
• Acute toxicities
• Late toxicities
Materials and Methods
It is a prospective randomized trial conducted on outdoor and indoor patients of Department of Radiotherapy and Clinical Oncology, JNMCH, AMU, ALIGARH enrolled during the period of October 2018 to November 2020.
Patient selection Inclusion criteria:
• Histologically confirmed ovarian carcinoma
• Age>18 years
• Karnofsky performance status (KPS) of >60
• Adequate hematologic (Hb>10 gm/dl, WBC>4000/l and platelets >1lakh/microlitre), Renal (serum creatinine <1.4 mg/dl) and Hepatic (serum bilirubin <1 mg/dl) function.
• No previous radiotherapy or chemotherapy
• Measurable tumor mass
• Unresectable , non-metastatic disease
Exclusion criteria:
• Patients who refuse to give consent
• Serious concomitant disease
• History of any prior or concurrent cancer in the last 5 years
• Pregnancy or breastfeeding
• Prior chemotherapy or radiotherapy
Pre treatment evaluation
• History and physical examination
• Measurement of detectable mass by physical examination
• Chest X-Ray
• CT scan abdomen and pelvis
• Blood cell count with differential counts, liver function studies, blood urea nitrogen and serum creatinine
• Serum CA125 as the tumor marker
Study design
• Prospective randomized study
• Written informed consent was obtained from all patients
• Patients were evaluated for response to treatment as well as for acute and late toxicities after completion of treatment according to the study protocol
TREATMENT PROTOCOL
Control arm
Received conventional neoadjuvant chemotherapy of Carboplatin administered at a dose of AUC 5 based on the Calvert formula18 using an EDTA glomerular filtration rate or AUC 6 based on a calculated creatinine clearance. Paclitaxel was administered at the dose of 175 mg/m2.The regimen was repeated every 21 days.
Study arm
Received dose dense weekly regimen of Paclitaxel administered at dose of 80 mg/m2 with Carboplatin administered three weekly at an AUC of 5.
Randomization
Patients satisfying inclusion criteria were randomized into two treatment arms by computer generated random table number.
Premedication administration
Our approved premedication for the 3-weekly regimen consisted of dexamethasone 16 mg, 12 h and 6 h before paclitaxel administration, diphenhydramine (or its equivalent) 50 mg i.v. 30 min-60 min before paclitaxel, and cimetidine 300 mg or ranitidine 50 mg i.v. 30-/60 min before paclitaxel.
When paclitaxel is given on a weekly basis, however, various premedication regimens have been used, many of which have used lower doses of dexamethasone to avoid problems with the corticosteroid intake.
Our patients of the dose dense arm were given dexamethasone 16 mg i.v., ondansetron 8 mg i.v. cimetidine 200 mg i.v. and diphenhydramine (or its equivalent) 50 mg i.v. 30 min-60 min before paclitaxel.
Chemotherapy administration
Conventional neoadjuvant chemotherapy included carboplatin administered at a dose of AUC 5 based on the Calvert formula 18 using an EDTA glomerular filtration rate or AUC 6 based on a calculated creatinine clearance. Paclitaxel was administered at the dose of 175 mg/m2 over 4 hrs-5 hrs via codon set .The regimen was repeated every 21 days.
Dose dense weekly regimen of Paclitaxel was administered at dose of 80mg/m2 with Carboplatin administered three weekly at an AUC of 5 or 6 as in the control group.
The chemotherapy was administered with proper premeditations and assessment of hydration status with preassessment of patient GC and vitals.
Assessment
Response
Response to treatment was evaluated by using the Gynaecological Cancer Intergroup response criteria, [18,19] and the Response Evaluation Criteria In Solid Tumors(RECIST), using both serum CA-125 and radiological criteria along with clinical assessment.
Acute and late toxicities
Patients were assessed for treatment related toxicities using Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
Follow up
1st follow up was done at one month of completion of treatment. The patient was sent for surgery immediately once the effect of neoadjuvant chemotherapy was seen. Subsequent long term follow up was done monthly for 3 months, then 2 monthly for next 6 months, then 3-4 monthly afterwards; but their results were not analyzed at time of this study interim analysis because we targeted the effect of dose dense chemotherapy regimen as NACT only till patient was taken up for surgery, due to our limited study period.
Observation And Results
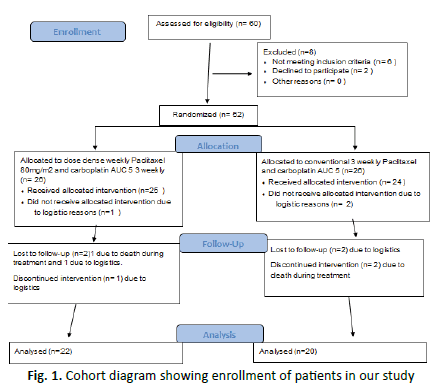
• It was a prospective randomized study conducted on diagnosed and untreated patients of advanced ovarian carcinoma registered in Department of Radiotherapy and Clinical Oncology, JNMCH, AMU, Aligarh between November 2018 to July 2020 (Figure 1).
Figure 1: Cohort diagram showing enrollment of patients in our study
• All patients of study arm received a minimum of 9 cycles of weekly paclitaxel with 3 cycles of three weekly carboplatin while all the patients of control arm received a minimum of 3 cycles of each paclitaxel and carboplatin
• The efficacy of NAC was evaluated at only one point, because surgery was performed immediately if chemotherapy was found to be effective
PATIENT CHARACTERISTICS
Age at diagnosis
Most of the patients who presented to us at diagnosis were of more than 60 years (46% in study group and 50% in control arm); while 36% study arm and 30% control arm belonged to 40-60 years age group. The mean age of patients in the study arm was 55.36 years (95% CI=55.36±5.45) with the median age being 59.5 years; SD was 13.044. While in the control arm the mean age was 51.15 years (95% CI=51.15±6.739) with median being 53 years; SD was 15.37. Both arms were balanced for age characteristics.
Menstrual and obstetric history
Majority of women belonged to the post-menopausal age group (73% in study arm and 90% in the control arm). Also most of the patients were multiparous with 3 or more children.
Performance status
In both the arms majority of patients had a satisfactory to good ECOG status, 64% in study arm and 70% in control arm having ECOG of 0-1;while 36% in study arm and 30% of control arm had ECOG of 2-3
Contraceptive history
Among the patients selected for study group only 2 out of 11 gave a history of OCP use while none among the control group had a positive history of OCP use.
TUMOR CHARACTERISTICS
Stage of disease at presentation
In the study arm, maximum patients presented with FIGO stage 3(46%) or stage 2(27%) disease. While in the control arm maximum presented with stage 3 disease (40%) followed by stage 1c (30%). Both arms were balanced with regards to stage of disease.
Tumour histology
Maximum detected histology of tumour was serous type in both the arms (46% in study arm and 40% in control arm). 27% in study arm and 20% in control arm were of mixed histology.10% in the control arm also represented unspecified histology.
CA-125 at diagnosis
Majority patients in both the arms had a baseline pre-treatment level of serum CA-125 between 501 U/ml-1000 U/ml.( 55% in study arm and 50% in control arm). The mean CA-125 at diagnosis in the study arm was 1304.5 U/ml (SD-1402.89; 95% CI=1304.5 ± 770.475) with median being 826 U/ml. While in the control arm the mean CA-125 at diagnosis was 1297.3 U/ml with median being 605.5U/ml(SD-1608.23; 95%CI=1297.3 ± 704.842).
RESPONSE ASSESSMENT
Response assessment was done 1 month after completion of last cycle of chemotherapy, i.e, after 6 cycles of 3 weekly standard regimen in control arm and 18 cycles of weekly paclitaxel with 6 cycles of three weekly carboplatin in the study arm. It was assessed according to RECIST 1.1 and GCIG criteria.
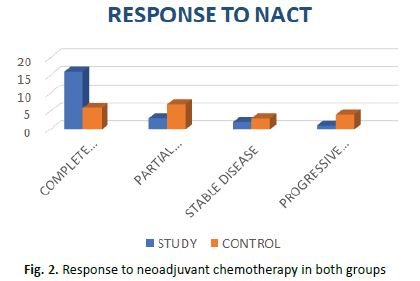
Complete response was seen in 72.9% of study arm and 30% of control arm patients. 13.6% patients in the study arm and 35% in the control arm showed partial response. While 4.5% of study arm and 20% of control arm patients showed progressive disease. Stable disease was seen in 9% of study arm and 15% of control arm patients. The result was significant at p=0.04 (Figure 2).
Figure 2: Response to neoadjuvant chemotherapy in both groups
Ca-125 response assessment
The date when the CA 125 level is first reduced by 50% is the date of the CA 125 response. To calculate response rates, an intent-to-treat analysis was used that includes all patients with an initial CA 125 level of at least twice the upper limit of normal as eligible and evaluable. Those patients who have both a CA 125 response and whose CA 125 level falls to within the normal range, can be classified as CA 125 complete responders. Patients who have a fall of CA 125 to within the normal range but whose initial CA 125 was less than twice the upper limit of normal, have not had a CA 125 response and cannot therefore be classified as a CA 125 complete responder. We evaluated CA- 125 response as per GCIG criteria after 1 month of the last cycle of chemotherapy (Table 1).
|
CA 125 RESPONSE |
STUDY ARM |
CONTROL ARM |
|
>50% Decrease and Normalized |
17 |
9 |
|
>50% Decrease but not Normalized |
3 |
7 |
|
Normalized but not >50% Decrease |
2 |
4 |
|
Progression |
0 |
0 |
Tab. 1. Ca-125 Response To Neoadjuvant Chemotherapy In both groups of patients of ovarian cancer
OVERALL DURATION OF NACT
It was calculated as the time from day 1 of first cycle of chemotherapy to the last day of last cycle of chemotherapy after which the patient was referred for surgery. The mean duration of neoadjuvant treatment in the dose dense weekly arm was 136.81 days (SD-6.19; 95%CI=136.81 ± 2.588) with the median duration being 135.5 days. In the conventional 3 weekly arm the mean duration of neoadjuvant treatment was 139.75days(SD-7.26; 95%CI=139.75 ± 3.182) with the median being 139.5 days.
TREATMENT DELAY
The chemotherapy cycle was interrupted due to grade 2 or above adverse effects / toxicities thus resulting in treatment delay in receiving the chemotherapy cycle. Almost every patient in both the arms showed a treatment delay. While in the dose dense weekly study arm 36.4% showed a delay in treatment within 1 week and 63.6% showed a delay of more than 1 week . In the conventional arm 30% showed delay within 1 week and 70% showed delay of more than 1 week.
Time to surgery
This was the duration of time elapsed between the last cycle of neoadjuvant chemotherapy and the day patient underwent interval debulking surgery. The mean time to surgery in the weekly dose dense arm was 70.09 days(SD-8.262; 95%CI=70.09±3.453) with median being 68 days. While in the conventional 3 weekly arm the mean time to surgery was 69.9days (SD-8.75; 95% CI=69.9 ± 3.839)with median being 66 days.
ACUTE TOXICITY ASSESSMENT DURING TREATMENT
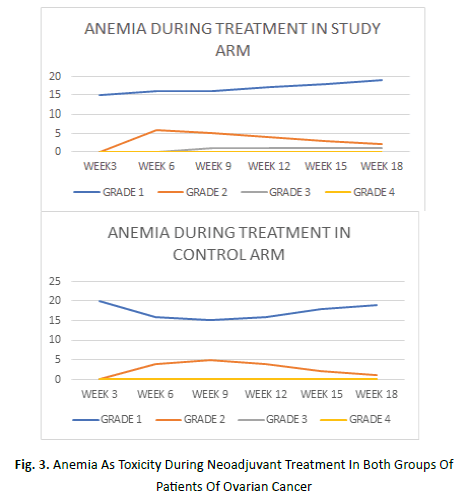
The onset of anemia in both the arms was seen as early as on week 3 of treatment. In the study arm it was maximum on week 18 while in the control arm it was maximum on week 3 itself. Grade 1 anemia was seen in an average of 77% patients in the study arm and 85% in control arm. Grade 2 anemia manifested in average 18% of study arm and 15% of control arm while grade 3 anemia was seen in average 9% of study arm (Figure 3).
Figure 3: Anemia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
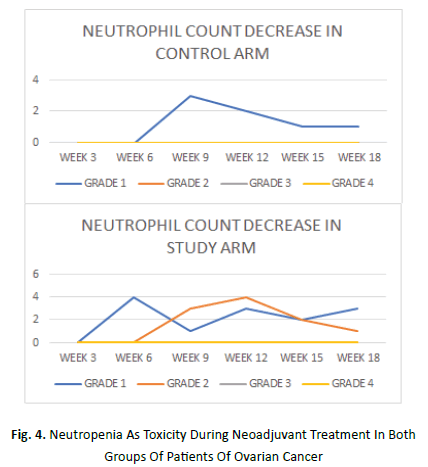
Onset of neutrophil count decrease in study arm manifested as early as week 3 while it began in control arm at week 6. It was maximum at week 6 followed by week 12 and week 18 in the study arm while the same in the control arm was at week 9. Grade 1 neutrophil count decrease was seen in average 9% patients of study arm and 5% patient of control arm. An average of 9% patients in study arm showed grade 2 decrease in neutrophil count. None of the patients in either arms showed grade 3 or 4 decrease in neutrophil count (Figure 4).
Figure 4: Neutropenia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
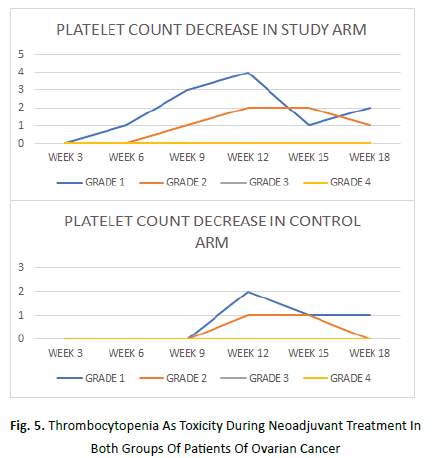
Platelet count decrease began as early as on week 3 in study arm while its onset was at week 9 in the control arm.It was maximum at week 12 in both the arms.An average of 9% patients in study arm and 5% patient in control arm manifested grade 1 platelet count decrease. Grade 2 platelet count decrease was seen in average 5% patient each of study and control arms. Grade 3 or 4 platelet count decrease was not seen in any patient of either arm.
During chemotherapy administration, acute reactions to paclitaxel infusion was also recorded. These allergic reactions were mostly transient and grade 1 (average of 9% patients of study arm and 5% patient in the control arm) which were seen as early as on week 3 in study arm and at week 6 in the control arm. Grade 2 allergic reaction was seen in average 5% patient of study arm and none of control arm (Figures 5 and 6).
Figure 5: Thrombocytopenia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
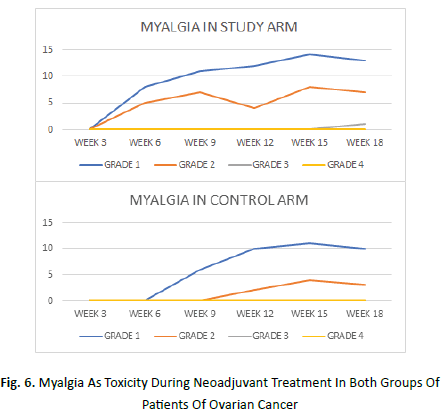
Figure 6: Myalgia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
Myalgia began at week 3 in the study arm and week 6 in the control arm.It was maximum at week 15 in both the arms. An average of 45% patients of study arm and 30% patients in the control arm manifested grade 1 myalgia. 23% patients in study arm and 10% patients in control arm showed grade 2 myalgia.
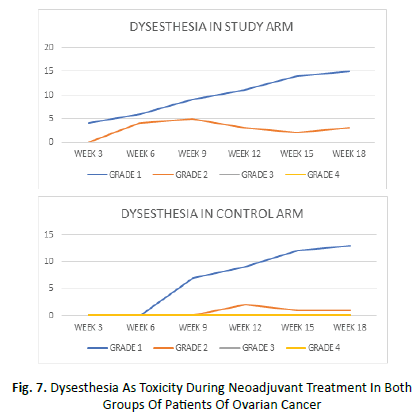
Dysesthesia began at week 3 in the study arm and week 6 in the control arm. It was maximum at week 15 in both the arms. Grade 1 dysesthesia was seen in average of 45% patients of study arm and 35% patients of control arm. An average of 14% patients in study arm and 5% patient of control arm manifested grade 2 dysesthesia. None of the patients showed grade 3 or 4 dysesthesia in either of the arms (Figure 7).
Figure 7: Dysesthesia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
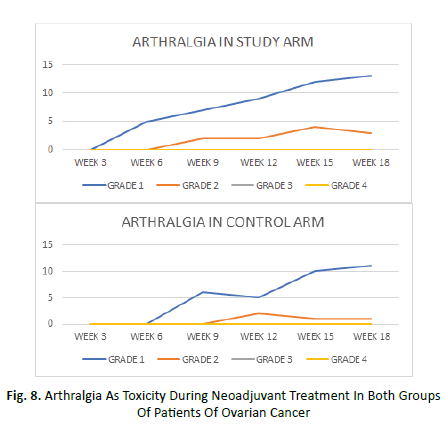
Arthralgia began at week 3 in the study arm and week 6 in the control arm and it was maximum at week 15 in both the arms. An average of 36% of patients in study arm and 25% patients in control arm manifested grade 1 arthralgia during treatment. Grade 2 arthralgia was seen in average 9% of study arm and 5% of control arm patient. Grade 3 or 4 arthralgia was not seen in any patient of either arms (Figure 8).
Figure 8: Arthralgia As Toxicity During Neoadjuvant Treatment In Both Groups Of Patients Of Ovarian Cancer
TOXICITIES AT 1 MONTH POST TREATMENT
On follow up at 1 month, anemia was present in around 90% of patients in both the arms. Grade 1 anemia was seen in 55% patients of both arms while grade 2 anemia was seen in 36% patients of study arm and 35% patients of control arm. Myalgia was seen in all patients of both arms at 1 month post treatment. Grade 1 myalgia was seen in 82% of study arm and 80% patients of control arm. Grade 2 myalgia manifested in 18% of study arm and 20% of control arm patients. Almost all patients also manifested dysesthesia in both the arms. Grade 1 dysesthesia was seen in 77% of study arm and 80% of control arm patients. While 23% study arm and 20% control arm patients presented with grade 2 dysesthesia. Almost all patients of study arm also manifested paraesthesias, grade 1 in 63.6% and grade 2 in 36.4 % patients. On the other hand, 90% patients in control arm manifested paraesthesias, grade 1 being 55% and grade 2 being 35% patients.
QUALITY OF LIFE ASSESSMENT
QoL was assessed using the validated patient selfreported questionnaires
Functional Assessment of Cancer Therapy (FACT)-general (FACT-G) and FACT-Ovary subscale.
The FACT questionnaires use a five-point response scale
0 referred to not at all; 1, a little bit; 2, somewhat; 3, quite a bit; and 4, very much. The range of possible scores was 0-108 for FACT-G and 0-44 for FACT-Ov. In FACT-G, the range of possible scores for the physical, social, emotional, and functional subscales were 0-28, 0-28, 0-24, and 0-28, respectively.
Patients were asked to complete the QoL assessment at three time points: baseline (before treatment), after the third and sixth chemotherapy cycles. At the third and sixth cycle, patients were given questionnaires at day 15 of completing the cycles.To assess QoL, scores were computed if more than 80% of items were answered in the overall and more than 50% of items in the FACT-Ov, and FACT-G subscales (Table 2).
|
Grade |
Physical well being | Social/ Family well-being | Emotional well- being | Functional well-being | Ovarian subscale | |
| Positive concerns | Negative concerns | |||||
| Good | 0-7 | >21 | 0-6 | >21 | >15 | 0-7 |
| Satisfactory | 14-Aug | 15-21 | 12-Jul | 15-21 | 15-Nov | 14-Aug |
| Poor | 15-21 | 14-Aug | 13-18 | 14-Aug | 10-Jun | 15-21 |
| Very poor | >21 | 0-7 | >18 | 0-7 | 0-5 | >21 |
Tab.2. Scoring scale used to assess various parameters in Quality of Life assessment
The following scoring scale was used to assess the various parameters.
In or study,the physical and functional well-being scale and ovarian subscale showed 1 unit increase in the study group in 1-2 patients at the end of 6th cycle .
Discussion
The general purpose of neoadjuvant chemotherapy is to reduce the tumour size or extent of cancer spread before applying the radical main treatment, thus making the procedure easier or less invasive. It also provides the chance to know whether the chemotherapy is effective, which is not possible a fter the tumour is completely removed.
In ovarian cancer, primary debulking surgery followed by adjuvant chemotherapy is a gold standard procedure. Although some investigators reported their favourable experience of neoadjuvant chemotherapy followed by interval debulking surgery, meta-analysis suggested that neoadjuvant chemotherapy was associated with poorer outcome. EORTC55971 was the first prospective randomized study of advanced (stage IIIC or IV) ovarian carcinoma, fallopian tube carcinoma, or primary peritoneal carcinoma to compare overall survival between patients who received standard primary debulking surgery followed by chemotherapy and those who received neoadjuvant chemotherapy plus interval debulking surgery. The majority of patients who entered this trial had extensive stage IIIC or IV disease at the treatment. The largest residual tumour 1 cm or less in diameter was achieved in 41.6% of patients after primary debulking and in 80.6% of patients after interval debulking.
Complete resection of all macroscopic disease (at primary or interval surgery) was the strongest independent variable in predicting overall survival.
Postoperative rates of adverse events and mortality were higher after primary debulking than after interval debulking. The CHORUS trial and JGOG0206 trials were similar trials addressing the role of neoadjuvant treatment in advanced ovarian cancer.
In our study in the dose dense weekly arm, maximum patients presented with FIGO stage 3(46%) or stage 2(27%) disease . While in the conventional 3 weekly arm maximum presented with stage 3 disease (40%) followed by stage 1c (30%). The basis of stage 1c and stage 2 patients being taken up for neoadjuvant therapy in our study was that either the patient was unfit for or unwilling to undergo upfront surgery.
The theoretical basis for dose-dense chemotherapy strategy is derived from Norton–Simon's model (Norton, 2001). Based on this concept, the increase of dose density should improve chemotherapy efficacy, minimizing the opportunity for regrowth of tumour cells between cycles of chemotherapy. Accordingly, several anticancer agents, such as paclitaxel, have an inhibitory effect on angiogenesis when given weekly. In fact, the effect on endothelial cell proliferation and migration is achieved with lower doses but requires a more frequent administration as endothelial cells recover already within 3-4 days after delivered chemotherapy. Correspondingly, in several cancer types, including breast and lung carcinomas, weekly administration has been proven effective and has been integrated in clinical practice.
Several preclinical studies support the rationale for weekly schedules of paclitaxel in ovarian cancer. The duration of exposure is an important determinant of the cytotoxic activity of paclitaxel. The dose-dense schedule leads to frequent exposure of tumor cells. Adequate cytotoxicity can be achieved at relatively low concentrations of paclitaxel provided that exposure is prolonged. Fennelly et al reported a phase I dose escalating study of weekly administration of paclitaxel alone in 18 ovarian cancer patients. Increasing the weekly dose of paclitaxel to100mg/m2 did not increase the nadir neutrophil counts to grade 3 or above, and a dose of 80 mg/m2 was recommended. Furthermore, they did not observe severe toxicity and hypersensitivity reactions commonly associated with paclitaxel administration. Responses were observed in 30% (4/13) of patients also including the patients with progressive disease after treatment with standard tri-weekly paclitaxel administration. It also conducted a phase I trial, administering paclitaxel over 1 h per week to breast and ovarian cancer patients; they also recommend a dose of 80 mg/ m. There were several phase II clinical trials using dose-dense weekly administration of paclitaxel and carboplatin, and those have demonstrated promising efficacy and favorable tolerability in patients with ovarian cancer. Weekly schedules of paclitaxel at a dose of 80 mg/m2 and carboplatin AUC 2 in a Japanese phase II study for recurrent ovarian cancer patients achieved a response rate of 67% (22/33). The hematologic and nonhematologic toxicities were tolerable. Grade 3 or 4 leukopenia and neutropenia was observed in 25% and 57%, respectively. In ovarian cancer, the Japanese Gynecologic Oncology Group (JGOG) first demonstrated the survival advantage of dose-dense weekly administration of paclitaxel. In JGOG3016, 637 patients were randomly assigned to receive six cycles of either paclitaxel (180 mg/m2 3-hour intravenous infusion) plus carboplatin (area under the curve 6 mg/mL/min), administered on day 1 of a 21-day cycle (conventional regimen; n=320), or dosedense paclitaxel(80 mg/m2 1 hour IV infusion) administered on days 1, 8, and 15 plus carboplatin administered on day 1 of a 21-day cycle (dose-dense regimen; n=317) which was same as in our study. The primary end point was progression-free survival, and secondary end points were overall survival and toxicity. Median progression-free survival was significantly longer in the dose-dense treatment group (28.0 months; 95% CI, 22.3 to 35.4 months) than in the conventional treatment group (17.2 months; p=0.0015). Overall survival at 3 years was higher in the dose-dense regimen group (72.1%) than in the conventional treatment group (65.1%; HR=0.75: p=0.03). Early discontinuation of the treatment was more frequent in dose-dense group than in the conventional group primarily because of the toxicities. On the basis of these results, it was concluded that dose-dense weekly paclitaxel plus carboplatin improved survival compared with the conventional triweekly administration of paclitaxel with the cost of modest increase in toxicities. The result of this trial has markedly influenced the designs of further clinical trials.
Very few trials have up-frontly addressed the role of efficacy of dose dense chemotherapy in neoadjuvant setting of ovarian cancer. Our study was thus conducted with the aim of addressing this aspect.
In a trial by testing the efficacy and safety of dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube or peritoneal cancer ,a response rate of 92% to dose dense taxane therapy as NAC was observed and disease progression did not occur in any patient. (Complete response 1 (4%) Partial response 22 (88%) Stable disease 2 (8%)). Another retrospective study by [17-25] for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer used the following two NAC regimens- (1) dose-dense TC: paclitaxel (80 mg/m2 on days 1, 8, or 15) and carboplatin (area under the curve [AUC], 6 on day 1; every 3 weeks) and (2) TC: paclitaxel (175 mg/m2 on day 1) and carboplatin (AUC, 6 on day 1; every 3 weeks),similar to our study.The response rate of NAC in the dose-dense TC and conventional-regimen groups was 92.1% [12,26-39] complete response (CR); 81, partial response (PR); and 8, stable disease (SD) or progression disease (PD)] and 92.3% (5, CR; 67, PR; and 6, SD or PD), respectively (p=0.47). In our study we observed that complete response was seen in 72.9% of study arm and 30% of control arm patients.13.6% patients in the study arm and 35% in the control arm showed partial response. While 4.5% of study arm and 20% of control arm patients showed progressive disease. Stable disease was seen in 9% of study arm and 15% of control arm patients. The result was significant at p=0.04.
As far as the toxicities are concerned; the landmark JGOG3016 trial observed that the most common adverse event was neutropenia (dose-dense regimen, 286 [92%] of 312 patients; conventional regimen, 276 [88%] of 314 patients, not statistically significant). The frequency of grade 3 and 4 anemia was significantly higher in the dose-dense group (n=214 [69%]) than in the conventional treatment group (n=137 [44%]; p=0.0001). The frequencies of other toxicities including peripheral neuropathy were similar between groups. In our study, during treatment, anemia was the most common adverse hematological event. Grade 1 anemia was seen in an average of 77% patients in the study arm and 85% of control arm. Grade 2 anemia manifested in average 18% patients of study arm and 15% patients of control arm Grade 3 anemia was seen in average 9% patient of study arm. Grade 1 neutrophil count decrease was seen in average 9% patients of study arm and 5% patient of control arm. An average of 9% patients in study arm showed grade 2 decrease in neutrophil count. The lower rates of neutropenia in our study might be contributed to the administration of prophylactic G-CSF to all patients in both the arms. An average of 9% patients in study arm and 5% patient in control arm manifested grade 1 platelet count decrease. Grade 2 platelet count decrease was seen in average 5% patient each of study and control arms. None of the patients manifested grade 3 or 4 neutropenia or thrombocytopenia during treatment in our study.
This is comparable and even lower than the earlier mentioned Japanese phase 2 study. The MITO-7 trial which used a lower dose of weekly paclitaxel(carboplatin (AUC 2 mg/mL per min) plus paclitaxel (60 mg/m²)) also showed toxicity results as follows- Fewer patients assigned to the weekly group than those allocated treatment every 3 weeks had grade 3-4 neutropenia (167 [42%] of 399 patients vs 200 [50%] of 400 patients), febrile neutropenia (two [0·5%] vs 11 [3%]), grade 3-4 thrombocytopenia (four [1%] vs 27 [7%]), and grade 2 or worse neuropathy (24 [6%] vs 68 [17%]).
The ICON-8 trial where stage IC-IV epithelial ovarian cancer were randomly assigned to group 1 (carboplatin area under the curve [AUC]5 or AUC6 and 175 mg/m2 paclitaxel every 3 weeks), group 2 (carboplatin AUC5 or AUC6 every 3 weeks and 80 mg/m² paclitaxel weekly), or group 3 (carboplatin AUC2 and 80 mg/m2 paclitaxel weekly). They observed that Grade 3 or 4 toxic effects were reported in 213 (42%) patients in group 1, 320 (62%) patients in group 2, and 269 (53%) patients in group 3. The major contributor to the higher incidence of grade 3 or higher events in both weekly treatment groups was uncomplicated neutropenia, which occurred in 76 (15%) of 508 patients in group 1, 181 (35%) of 513 patients in group 2, and 152 (30%) of 510 patients in group 3. The incidence of febrile neutropenia was low across all three groups with 21 (4%) in group 1, 31 (6%) in group 2, and 16 (3%) in group 3, with no significant difference between groups 2 or 3 and group 1 However, the incidence of grade 3 or higher anaemia was significantly higher in patients assigned to group 2 compared with those receiving standard 3-weekly chemotherapy (25 [5%] in group 1 vs 65 [13%]in group 2;p<0·0001]) but not in patients assigned to group 3 (24 [5%], p=1·00).
Similar outcomes were observed in our study. We evaluated toxicity during treatment at weeks 3,6,9,12,15 and 18. All grades individually reported were considered and the average number of patients manifesting that grade were reported. All toxicity assessment results were statistically non-significant(p value>0.05).
In a study by [4,40-55] randomly assigned ovarian cancer patients of stage 3 or 4 to receive either paclitaxel, administered intravenously at a dose of 175 mg per square meter of bodysurface area every 3 weeks, plus carboplatin (dose equivalent to an area under the curve [AUC] of 6) for six cycles or paclitaxel, administered weekly at a dose of 80 mg per square meter, plus carboplatin (AUC, 6) for six cycles similar to our study. They reported that the most common adverse events of grade 3 or higher were neutropenia (in 78% of the patients), gastrointestinal disorders (in 21%, including 2% who had gastrointestinal-wall disruption, such as perforation, fistula, or necrosis), thrombocytopenia (in 18%), infection (in 4%), and anemia (in 26%) . Anemia of grade 3 or higher was reported in 36% (124 of 340 patients) of the patients who received weekly paclitaxel, as compared with 16% of those (54 of 343) treated with paclitaxel every 3 weeks (P<0.001). However, neutropenia of grade 3 or higher occurred less often in the group that received weekly paclitaxel than in the group that received paclitaxel every 3 weeks (72% [246 of 340 patients] vs. 83% [286 of 343], P<0.001). There was no significant between-group difference in the incidence of sensory neuropathy of grade 3 or higher, more patients in the group that received weekly paclitaxel than in the group that received paclitaxel every 3 weeks had sensory neuropathy of grade 2 or higher (26% [88 of 340 patients] vs. 18% [61 of 343] .
We did not assess gastrointestinal toxicities in our study because during our limited time frame and with limited patient size, almost all patients presented with GI complaints at diagnosis, during and after treatment. Our limited follow up had the drawback of being unable to distinguish the GI symptoms to be due to disease per se or as an adverse effect to neoadjuvant treatment.
In our patients, myalgia began at week 3 and week 6 respectively in the study and control arms, being maximum at week 15 in both .An average of 45% patients of study arm and 30% patients in the control arm manifested grade 1 myalgia. An average of 23% patients in study arm and 10% patients in control arm showed grade 2 myalgia. None of the patients in either arm manifested grade 3 or 4 myalgia. Grade 1 dysesthesia was seen in average of 45% patients of study arm and 35% patients of control arm. An average of 14% patients in study arm and 5% patient of control arm manifested grade 2 dysesthesia. An average of 36% of patients in study arm and 25% patients in control arm manifested grade 1 arthralgia during treatment. Grade 2 arthralgia was seen in average 9% of study arm and 5% of control arm patient.
In a retrospective case-control study by chemotherapy induced peripheral neuropathy among patients with ovarian cancer receiving taxanes- out of 88 women included in the study 61(69.3%) reported CIPN. Twelve months after chemotherapy, it was 19.3%. The percentage of patients suffering from sensory peripheral neuropathy was higher than motor neuropathy at any time during their study. SPN was associated with the use of docetaxel and paclitaxel: (docetaxel vs liposomal paclitaxel OR 4.39(95% CI 1.69-11.42,p=0.019); (paclitaxel vs liposomal paclitaxel; OR-5.91,95%CI=1.09-31.97,p=0.04). They thus concluded that significant proportion of patients with ovarian cancer receiving taxanes suffered from long term residual neuropathy, mostly sensory neuropathy.
Paclitaxel induces a progressive, predominantly sensory neuropathy. Symptoms can occur after the first dose, and include painful paresthesia as well as numbness of the hands and feet. Transient myalgia is common after each dose, which usually resolves within days. Sensory loss presents in a stocking-glove distribution. Ankle jerks and other reflexes may be diminished or absent, which progresses with cumulative doses. Both small and large fiber sensory functions are affected. Muscle strength is frequently preserved or only minimally affected. Overall, either single or cumulative dose is the most important factor to consider in taxanes-induced neuropathy. Most symptoms usually improve or resolve after discontinuation of treatment, however, severe symptoms may persist for a long period of time. The taxanes block tubulin depolymerisation, leading to the inhibition of microtubule dynamics and cell cycle arrest. In patients, paclitaxel produced early sensory dysfunction in 4 weeks as increasing in stimulus threshold and reduction in sensory amplitudes on neurophysiological and nerve excitability studies; 71% of patients developed symptoms by 6 weeks after administration of about 500 mg/m2. Reduced sensory amplitudes or abolishment of sensory responses on neurophysiology studies were also found in patients treated with docetaxel. Carboplatin induced neuropathy has similar symptoms to those of cisplatin, but absent Lhermitte sign. The neurotoxicity of carboplatin is generally considered to be less frequent, and less severe than cisplatin. Grade 3/4 sensory neuropathy was 13.5% in the cisplatin regimen versus 7.2% in the carboplatin regimen [56-59].
In our study the onset of neuropathic symptoms began as early as on week 3 in dose dense arm an week 6 in the conventional arm. A longer follow up is required to assess the actual results due to cumulative doses and other factors. We assessed neuropathic toxicity as per CTCAE v4.03 which was symptom based . But the above mentioned studies evaluated the same using EORTC quality of life questionnaire based method. This is a discrepancy to be noted.
In our study, The mean duration of neoadjuvant treatment was 136.81 days and 139.75 days respectively in study and control groups. All patients in both the arms showed a treatment delay with 36.4% showing a delay in treatment within 1 week and 63.6% a delay of more than 1 week in study arm . In the conventional arm the same were 30% and 70% respectively. The mean time to surgery in the study arm was 70.09 days and 69.9days in control arm.
In a study by [60-66] al -Comparison between weekly versus 3-weekly paclitaxel in combination with carboplatin as neoadjuvant chemotherapy in advanced ovarian cancer- Patients in the dose dense and standard group both received a median of 3 NAC cycles (p=0.377). One patient in the dose dense group received interval debulking surgery after 2 NAC cycles due to severe ascites and exacerbation of underlying comorbidities. Dose was reduced during NAC in 8 patients (34.8%) in the dose dense group and in 2 patients (4.0%) in the standard group (p=0.001). Events of grade III and higher neutropenia were observed more often in the dose dense group than in the standard group (82.6% vs. 22.0%, p<0.001). In the dose dense group, 12 patients (52.2%) received red blood cell transfusion, whereas no patient received platelet transfusion. In total, 18 patients (85.7%) in the dose dense group and 14 patients (28.0%) in the standard group received at least 1 cycle of G-CSF replacement; yet there were no events of neutropenic fever. Rates of grade III and higher anemia and lymphopenia were not significantly different, and there was no event of grade III and higher thrombocytopenia. Other non-hematologic toxicities including infection, thrombosis, gastrointestinal, and fatigue were comparable between both groups.
Treatment delay of more than 1 week incurred in 3 patients (13.0%) in the dose dense group due to pancytopenia, whereas no patient in the standard group experienced treatment delay (p=0.028). The difference in the rate of hospitalization was not statistically significant between the 2 groups (17.4% in dose dense group vs. 4.0% in standard group, p=0.074). Indications for hospitalization in the dose dense group included infection (n=4), nausea and vomiting (n=1), and fatigue (n=1). One patient in the standard group was hospitalized for continuation of intravenous antibiotic treatment for infective spondylitis, and underlying diagnosis prior to NAC. All other hospitalized patients were admitted once during NAC and for duration of less than 1 week, and indications for hospitalization included non-neutropenic fever, gastrointestinal symptom, and fatigue. There were no events of small bowel obstruction and intraabdominal haemorrhage.
In our study, treatment delay of within 1 week did not involve any hospitalisation and was mostly due to hematologic toxicity; especially anemia requiring transfusion. Among those showing delays of more than 1week, indications for hospitalization in dose dense arm included pancytopenia(n=4), post chemotherapy nausea and vomiting(n=8) and generalised weakness (n=2) while in the conventional arm, hospitalization was indicated for febrile neutropenia in 1 patient, generalized weakness in 3 patients and post chemo nausea vomiting in 3 patients. All patients in our study received minimum of 6 cycles of NACT in both the arms( 18 cycles of weekly paclitaxel+ 6 cycles 3 weekly carboplatin in dose dense arm; 6 cycles of 3 weekly paclitaxel+ carboplatin in conventional arm) [67,68].
The NAC regimen was only specified as three cycles of platinum based chemotherapy in two Phase III studies (EORTC55971/ NCIC OV13 and CHORUS ) that confirmed non-inferiority of OS between IDS after NAC and PDS. In contrast, the NAC regimen was specified as four cycles of tri-weekly TC therapy in an ongoing Phase III study (JCOG0602) with a similar design (20).
There is currently no consensus about the optimal number of NAC cycles. In the JGOG3016 study, IDS could be performed after from two to four cycles of NAC. However, it was considered more appropriate to set three cycles of ddTC as NAC in the study by Tomoko Yoshihama, Hiroyuki Nomura et al.
Also in our study the mean time to surgery in the weekly dose dense arm was 70.09 days with median being 68 days. While in the conventional 3 weekly arm the mean time to surgery was 69.9days with median being 66 days. In a study by Ming Chen, Zhan peng Chen et al, Impact of the Time Interval from Neoadjuvant Chemotherapy to Surgery in Primary Ovarian, Tubal, and Peritoneal Cancer Patients. They evaluated the effect of the time interval between the end of NACT and surgery (TTS ≤ 4 weeks vs TTS > 4 weeks) on the survival outcomes among patients with advanced-stage ovarian, tubal, and peritoneal cancers. 152 patients with stage III or IV ovarian, tubal, and peritoneal cancers were included in this retrospective cohort study: 115 in the TTS ≤ 4 weeks and 37 in the TTS >4 weeks groups. The Kaplan-Meier analysis showed that the progressionfree survival in the TTS ≤4 weeks group was longer than that in the TTS >4 weeks group (26 vs 14 months, P=0.04). However, the overall survival was not different between the two groups (66 vs 36 months, P=0.105). The multivariate analysis presented that delay in surgery after NACT (TTS >4 weeks) was associated with a shorter progression-free (P=0.002) but not overall survival (P=0.231). Their findings demonstrated no relationship between the NACT to surgery interval and OS, while a detrimental effect of TTS >4 weeks on PFS was observed.
Our study needs a further longer follow up to assess overall survival and progression free survival reported as end points in earlier landmark studies.
Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II-IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016) by K.Harano et al concluded that dose dense therapy does not decrease overall QoL compared with conventional therapy . In the MITO-7 trial which also objected at QoL assessment as a primary end point it was observed that FACT-O/TOI scores diï¬?ered significantly between the two schedules (treatment-by-time interaction p<0·0001); with treatment every 3 weeks, FACT-O/TOI scores worsened at every cycle (weeks 1, 4, and 7), whereas for the weekly schedule, after transient worsening at week 1, FACT-O/TOI scores remained stable. Further studies prospectively directed at QoL assessment in dose dense chemotherapy in neoadjuvant ovarian cancer setting are lacking. In our study we also assessed Quality of life at 3 time points: baseline (before treatment), after the third and sixth chemotherapy cycles,. The physical and functional wellbeing scale and ovarian subscale showed 1 unit increase in the study group in 1-2 patients at the end of 6th cycle.
Limitations of Our Study
Small number of patients enrolled in our study is a drawback. Another limitation is our shorter follow up period that prevented in analysing late toxicities like peripheral neuropathy. As in previous standard studies with primary end point of evaluation being progression free survival and overall survival, we require a further longer follow up to analyse the same.
Conclusion
We aimed at analysing the efficacy ,tolerance and toxicity of weekly dose dense regimen of paclitaxel with three weekly carboplatin in comparison to standard 3 weekly regimen of paclitaxel and carboplatin, specially in the neoadjuvant setting for advanced ovarian cancer.
We found out significant overall response rate with the dose dense regimen and also the toxicities during treatment were tolerable. We aim to further continue our study to analyse survival outcome effects in the long run, which is the primary end-point of analysis in majority of landmark trials in this perspective. It can thus be concluded that weekly dose dense paclitaxel with carboplatin is an effective and feasible treatment option in neoadjuvant setting in patients with advanced ovarian cancer with good response rates and acceptable toxicities. Further prospective trials evaluating the efficacy and tolerability of dose dense paclitaxel is warranted.
References
- [Conte PF, Cianci C, Gaducci A. Up date in the management of advanced ovarian carcinoma. Crit Rev Oncol Hematol 1999;32:49-58.
[Google Scholar] [Cross-ref] - McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer. Semin Oncol 1998;25:340-348.
[Google Scholar] [Cross-ref] - Vermorken JB. The integration of paclitaxel and new platinum compounds in the treatment of advanced cancer. Int J Gynecol Cancer 2001;11(Suppl. 1):21-30.
[Google Scholar] [Cross-ref] - McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1-6.
[Google Scholar] [Cross-ref] - McGuire WP, Hoskins WJ, Brady MF, et al. Long-term followup of GOG-111: a randomized trial comparing cisplatin combined with cyclophosphamide or paclitaxel in patients with stage III or IV epithelial ovarian cancer. Int J Gynecol Cancer 1999;9(Suppl.1):8.
[Google Scholar] [Cross-ref] - Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst 2000;92:699-708.
[Google Scholar] [Cross-ref] - Seidman AD, Hudis CA, Albanel J, et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 1998;16:3353-3361.
[Google Scholar] [Cross-ref] - Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol 1997;24(Suppl. 10):S10.3/S10.10.
- Up date in the management of advanced ovarian carcinoma.
- Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 1996;2:1843-1849.
[Google Scholar] [Cross-ref] - Lau DH, Xue L, Young LJ, Burke PA, Cheung AT. Paclitexel (Taxol†): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 1999;14:31-36.
[Google Scholar] [Cross-ref] - Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol. 2012 Jan;124(1):10-4. Epub 2011 Sep 13.
[Google Scholar] [Cross-ref] - National Comprehensive Cancer Network . Ovarian Cancer (Version 2.2020),
[Google Scholar] [Cross-ref] - Martine J. Piccart, Kamma Bertelsen, Keith James, Jim Cassidy, Constantino Mangioni, et al, Randomized Intergroup Trial of Cisplatin–Paclitaxel Versus Cisplatin–Cyclophosphamide in Women With Advanced Epithelial Ovarian Cancer: Three-Year Results, JNCI: Journal of the National Cancer Institute, 2000;92(9):699-708
[Google Scholar] [Cross-ref] - Sabbatini P, Aghajanian C, Pezulli K, et al. Phase I study of paclitaxel and carboplatin in patients (pts) with advanced ovarian, peritoneal or fallopian tube cancer. Proc Am Soc ClinOncol 1998;17:A1441.
[Google Scholar] [Cross-ref] - Katsumata N, Watanabe T, Mukai H, et al. A phase II trial of weekly paclitaxel/carboplatin (TJ) as salvage chemotherapy in patients with relapsed ovarian cancer. Proc Am Soc Clin Oncol 2001;20:865
[Google Scholar] [Cross-ref] - Bolanos M, Borrega P, Gonzalez-Beca R, et al. Weekly paclitaxel/carboplatinum (P/CAR) as first-line chemotherapy in late relapses(LR) of epithelial ovarian cancer (OC). Preliminary results. ProcAm Soc Clin Oncol 2001:870.
[Google Scholar] [Cross-ref] - Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol 2013;14:1020-1026.
[Google Scholar] [Cross-ref] - Karst AM, Drapkin R. Ovarian cancer pathogenesis: a model in evolution. J Oncol 2010;2010:932371.
[Google Scholar] [Cross-ref] - Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 2008;26(32):5284-5293.
[Google Scholar] [Cross-ref] - Auersperg N, Wong AS, Choi KC, et al. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 2001;22(2):255-288.
[Google Scholar] [Cross-ref] - Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet 1971;2(7716):163.
[Google Scholar] [Cross-ref] - Casagrande JT, Louie EW, Pike MC,et al. “Incessantovulation” and ovarian cancer. Lancet 1979;2(8135):170-173.
[Google Scholar] [Cross-ref] - Fleming JS, Beaugie CR, Haviv I, et al. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis:revisiting old hypotheses. Mol Cell Endocrinol 2006;247(1-2):4-21.
[Google Scholar] [Cross-ref] - Choi JH, Wong AS, Huang HF, et al. Gonadotropins and ovarian cancer. Endocr Rev 2007;28(4):440-461.
[Google Scholar] [Cross-ref] - Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst 1983;71(4):717-721.
[Google Scholar] [Cross-ref] - Wright JW, Pejovic T, Fanton J, et al. Induction of proliferation in the primate ovarian surface epithelium in vivo. Hum Reprod 2008;23(1):129-138.
[Google Scholar] [Cross-ref] - Schlosshauer PW, Cohen CJ, Penault-Llorca F, et al. Prophylactic oophorectomy: a morphologic and immunohistochemical study. Cancer 2003;98(12):2599-2606.
[Google Scholar] [Cross-ref] - Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 2007;25(25):3985-3990.
[Google Scholar] [Cross-ref] - Tone AA, Begley H, Sharma M, et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res 2008;14(13):4067-4078.
[Google Scholar] [Cross-ref] - Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol 2013;24(Suppl 10):x16–x21.
[Google Scholar] [Cross-ref] - Yawn BP, Barrette BA, Wollan PC. Ovarian cancer: the neglected diagnosis. Mayo Clin Proc 2004;79(10):1277-1282.
[Google Scholar] [Cross-ref] - Griffiths CT. Surgical resection of tumor bulk in the primarytreatment of ovarian carcinoma. Natl Cancer Inst Monogr 1975;42:101-104.
[Google Scholar] [Cross-ref] - Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20(5):1248-1259.
[Google Scholar] [Cross-ref] - Mueller JJ, Zhou QC, Iasonos A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol 2016;140(3):436-442.
[Google Scholar] [Cross-ref] - Martin R. Platinum complexes: hydrolysis and binding to N(7) and N(1) of purines. In: Lippert B, ed. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Zurich: Verlag Helvetica Chimica Acta; 1999:183.
[Google Scholar] [Cross-ref] - Harrap KR. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev 1985;12(Suppl A):21-33.
[Google Scholar] [Cross-ref] - Calvert AH, Harland SJ, Newell DR, et al. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol 1982;9(3):140-147.
[Google Scholar] [Cross-ref] - Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function.J Clin Oncol 1989;7(11):1748-1756.
[Google Scholar] [Cross-ref] - Egorin MJ, Van Echo DA, Olman EA, et al. Prospective validation of a pharmacologically based dosing scheme for the cis -di-ammine-di-chloro platinum(II) analogue di-ammine-cyclo-butane-di-carboxylato platinum. Cancer Res 1985;45(12 Pt 1):6502-6506.
[Google Scholar] [Cross-ref] - . Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther 1987;34(2):155-166.
[Google Scholar] [Cross-ref] - Zhu G, Song L, Lippard SJ. Visualizing inhibition of nucleosome mobility and transcription by cisplatin-DNA inter-strand crosslinks in live mammalian cells. Cancer Res 2013;73(14):4451-4460.
[Google Scholar] [Cross-ref] - . Martens-de Kemp SR, Dalm SU, Wijnolts FM, et al. DNA-bound platinum is the major determinant of cisplatin sensitivity in head and neck squamous carcinoma cells. PLoS One 2013;8(4):e61555
[Google Scholar] [Cross-ref] - Duffull SB, Robinson BA. Clinical pharmacokinetics and dose optimization of carboplatin. Clin Pharmacokinet 1997;33(3):161–183.
[Google Scholar] [Cross-ref] - Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res 1984;44(4):1693–1697.
[Google Scholar] [Cross-ref] - Chatelut E, Canal P, Brunner V, et al.Prediction of carboplatinclearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 1995;87(8):573–580.
[Google Scholar] [Cross-ref] - Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients withovarian cancer. J Clin Oncol 1992;10(4):520–528.
[Google Scholar] [Cross-ref] - Reyno LM, Egorin MJ, Canetta RM, et al. Impact of cyclophosphamide on relationships between carboplatin exposure and response or toxicity when used in the treatment of advanced ovarian cancer. J Clin Oncol 1993;11(6):1156–1164.
[Google Scholar] [Cross-ref] - Ma J, Verweij J, Planting AS, et al. Current sample handling methods for measurement of platinum-DNA adducts in leucocytes in man lead to discrepant results in DNA adduct levels and DNA repair. Br J Cancer 1995;71(3):512–517.
[Google Scholar] [Cross-ref] - Schellens JH, Ma J, Planting AS, et al. Relationship between the exposure to cisplatin, DNA-adduct formation in leucocytes and tumour response in patients with solid tumours. Br J Cancer 1996;73(12):1569–1575.
[Google Scholar] [Cross-ref] - Omura, G., Blessing, J.A., Ehrlich, C.E., Miller, A., Yordan, E., et al. (1986), A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A gynecologic oncology group study. Cancer, 57: 1725-1730.
[Google Scholar] [Cross-ref] - Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer 2010;10(3):194–204.
[Google Scholar] [Cross-ref] - Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature.1979;277,665-667.
[Google Scholar] [Cross-ref] - Dai H, Ding H, Meng XW, et al. Contribution of Bcl-2 phosphorylation to Bak binding and drug resistance. Cancer Res2013;73(23):6998–7008.
[Google Scholar] [Cross-ref] - Vigano L, Locatelli A, Grasselli G, et al. Drug interactions of paclitaxel and docetaxel and their relevance for the design of combination therapy. Invest New Drugs 2001;19(2):179–196.
[Google Scholar] [Cross-ref] - Kudlowitz D, Muggia F. Defining risks of taxane neuropathy: insights from randomized clinical trials. Clin Cancer Res 2013;19(17):4570–4577.
[Google Scholar] [Cross-ref] - Sissung TM, Mross K, Steinberg SM, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer 2006;42(17):2893–2896.
[Google Scholar] [Cross-ref] - Abraham JE, Guo Q, Dorling L, et al. Replication of genetic polymorphisms reported to be associated with taxane related sensory neuropathy in patients with early breast cancer treated with Paclitaxel.Clin Cancer Res 2014;20(9):2466–2475.
[Google Scholar] [Cross-ref] - Baldwin RM, Owzar K, Zembutsu H, et al. A genome-wide association study identifies novel loci for paclitaxel induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 2012;18(18):5099–5109.
[Google Scholar] [Cross-ref] - Andreas du Bois, Thaïs Baert, and Ignace Vergote ; Role of Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer; Journal of Clinical Oncology 2019 37:27, 2398-2405
[Google Scholar] [Cross-ref] - Sean Kehoe, Jane Hook, Matthew Nankivell, Gordon C. Jayson, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: Results from the MRC CHORUS trial; Journal of Clinical Oncology 2013 31:15_suppl, 5500-5500
[Google Scholar] [Cross-ref] - Pignata, Sandro et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial; The Lancet Oncology, Volume 15, Issue 4, 396 – 405.
[Google Scholar] [Cross-ref] - Andrew R et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial; The Lancet, Volume 394, Issue 10214, 2084 – 2095.
[Google Scholar] [Cross-ref] - Chan J Brady M Penson R et al.;Phase III trial of every-3-weeks paclitaxel vs. dose dense weekly paclitaxel with carboplatin +/− bevacizumab in epithelial ovarian, peritoneal, fallopian tube cancer: Int J Gynecol Cancer. 2013; 23: 9.
[Google Scholar] [Cross-ref] - Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N Engl J Med. 2016 Feb 25;374(8):738-48. doi: 10.1056/NEJMoa1505067.
[Google Scholar] [Cross-ref] - Yoshihama T, Nomura H, Iwasa N, Kataoka F, Hashimoto S, et al. Efficacy and safety of dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube or peritoneal cancer. Jpn J Clin Oncol. 2017 Nov 1;47(11):1019-1023.. PMID: 28973541.
[Google Scholar] [Cross-ref] - Kim YN, Lee YJ, Lee JY, Nam EJ, Kim SW, et al. Comparison between weekly versus 3-weekly paclitaxel in combination with carboplatin as neoadjuvant chemotherapy in advanced ovarian cancer. J Gynecol Oncol. 2020 Mar;31(2):e23.
[Google Scholar] [Cross-ref] - Min Cheng, Howard Hao Lee, Wen-Hsun Chang, Na-Rong Lee, Hsin-Yi Huang, et al. (2019) Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. International Journal of Environmental Research and Public Health 16:23, 4794.
[Google Scholar] [Cross-ref] - Andrea Milani, Rebecca Kristeleit, Mary McCormack, Fharat Raja, Daniela Luvero, et al. Switching from standard to dose-dense chemotherapy in front-line treatment of advanced ovarian cancer: a retrospective study of feasibility and efficacy; ESMO Open, Volume 1, Issue 6, 2016,ISSN 2059-7029.
[Google Scholar] [Cross-ref]