Research Article - Onkologia i Radioterapia ( 2020) Volume 14, Issue 6
Comparative study between treatment related acute toxicity in patients receiving 3DCRT/IMRT as a definitive radiotherapy for localized prostatic carcinoma with assessment of quality of life in those patients
Shorouk G. Badr1, Samar Galal Younis1, Gamal Alhusiny Atia2 and Fatma Gharib1*2Department of Clinical Oncology and Nuclear Medicine, Egypt
Fatma Gharib, Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Egypt, Email: dfatma1980@yahoo.com
Received: 05-Sep-2020 Accepted: 22-Oct-2020 Published: 30-Nov-2020
Abstract
Comparative study between treatment related acute toxicity in patients receiving 3DCRT/ IMRT as a definitive radiotherapy for localized prostatic carcinoma with assessment of quality of life in those patients Shorouk G. Badr(1) , Samar Galal Younis(1), Gamal Alhusiny Atia(2), Fatma Gharib(1) (1) Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Tanta University (2) Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Alexandria University Background: Prostate cancer is a leading cause of mortality and morbidity globally. In Egypt, prostate cancer comes in the sixth rank. External beam radiation therapy (EBRT) is well-established line of treatment in prostate cancer. EBRT can be delivered by many modalities including 3DCRTand IMRT. IMRT offered less gastrointestinal and genitourinary side effects and hence better health related quality of life for many patients in several studies. Aim: Comparison between acute gastrointestinal, genitourinary, hematological toxicities, fatigue related to radiotherapy and health related quality of life in patients receiving 3D conformal radiotherapy and IMRT for localized /locally advanced prostatic cancer. Patients and methods: This prospective study was carried out at clinical oncology departments Tanta university hospitals and Alexandria university hospitals through the period from May 2018 to May 2019 and included 30 intermediate and high risk prostate cancer patients received 2 modalities of radiotherapy divided into 2 arms. Fifteen patients received 3D conformal radiotherapy concurrent with ADT (Arm A) and 15 patients received IMRT concurrent with ADT (Arm B). Results: IMRT resulted in significantly lower incidence of rectal pain and microscopic hematuria and dysuria and lower incidence of grade ≥ 2 diarrhea, proctitis, rectal pain and cystitis during radiotherapy compared to 3DCRT but with little impact in overall quality of life which was better in IMRT group but without statistical significance except 6 months after radiotherapy. Conclusion: IMRT may offer lower gastrointestinal and genitourinary radiation toxicity but with no or little difference in overall health related quality of life.
Keywords
prostate cancer, 3DCRT, IMRT, radiation toxicity, quality of life
Introduction
Prostate cancer remains the most common cancer and the third leading cause of cancer mortality in men [1]. Newly diagnosed men with localized prostate cancer have several treatment options that include watchful waiting, radical prostatectomy, radiotherapy (external beam or brachytherapy), hormonal ablation and a combination of these modalities [2]. External Beam Radiation Therapy (EBRT) is a principle treatment for prostate cancer (both localized and locally advanced). Doseescalation to the prostate was proved to enhance biochemical PFS; however, this can be on expense of more Gastrointestinal (GI) and Genitourinary (GU) adverse effects [3]
Radiotherapy related toxicities include gastrointestinal, GU toxicity, sexual dysfunction, hematological toxicity and fatigue. These treatment-related toxicities may be acute (typically within 3 months) or chronic and greatly affect quality of life.
Attempts to enhance the therapeutic range, particularly a reduction in treatment-related adverse effects, have made progress in modern RT techniques such as Intensity Modulated Radiation Therapy (IMRT) [4]. Moreover, IMRT has been shown to improve the local control and DFS in localized prostate cancer patients [5].
The main characteristic of IMRT in comparison with 3-dimensional conformal radiation therapy (3D-CRT) is the potential to escalate the dose of radiation to the prostate [6], while minimizing the radiation dose to neighbouring healthy tissues, including the rectum and bladder, therefore reducing treatment related toxicities [7].
Image Guided Intensity Modulated Radiotherapy (IGIMRT) has created a higher accuracy of the dose delivery and consequently decreased radiation toxicity [8].
Aim
Comparison between acute gastrointestinal, genitourinary, haematological toxicities, fatigue related to radiotherapy and health related quality of life in patients receiving 3D conformal radiotherapy and IMRT for localized/locally advanced prostatic cancer.
Patients and Methods
This prospective study was carried out at clinical oncology departments Tanta university hospitals and Alexandria university hospitals through the period from May 2018 to May 2019 and included 30 intermediate and high-risk prostate cancer patients received 2 modalities of radiotherapy divided into 2 arms. Fifteen patients received 3DCRT concurrent with ADT (Arm A) and 15 patients received IMRT concurrent with ADT (Arm B).
The following data was collected; careful history and Clinical examination with assessment of ECOG performance status, initial clinical TNM staging according to AJCC eighth edition 2017, categorization of patients according to prognostic risk grouping from the National Comprehensive Cancer Network (www.nccn.org) risk classification depending on T stage, Gleason score and pre-treatment serum PSA.
Inclusion criteria
Patients with non-metastatic prostatic adenocarcinoma, unfavourable intermediate, high risk and very high-risk criteria with ECOG performance status ≤ 2.
Exclusion criteria
Patients with metastatic prostate cancer, history of radical prostatectomy, pathology other than adenocarcinoma eg. (neuroendocrinal tumor, sarcoma or lymphoma), ECOG performance status >2.
Methods
Careful history and Clinical examination with assessment of ECOG performance status, initial clinical TNM staging according to AJCC eighth edition 2017, categorization of patients according to prognostic risk grouping from the National Comprehensive Cancer Network (www.nccn.org) risk classification depending on T stage, Gleason score and pre-treatment serum PSA were done.
Radiotherapy
A planning Computed Tomography (CT) simulation with (2-3 mm) slice thickness was done for all patients in a supine position with arms folded on the chest and out of field.
All patients were advised to empty bladder and drink 600 ml water (small bottle of water) 30 minutes and an enema to empty the rectum before CT simulation. Immobilization techniques were used during CT simulation and all treatment fractions. Fusion between planning CT and MRI was done (if available).
The primary Clinical Target Volume (CTV) included the whole prostate visible on CT and seminal vesicle base (1 cm), CTV nodal included pelvic lymph nodes (common iliac, internal iliac, external iliac, and pre-sacral lymph nodes). PTV margin 5-6 mm was added around CTV primary and CTV nodal.
Organs At Risk (OARs) including femoral heads, rectum, bladder, penile bulb and bowel were contoured following the recommendations of the Radiation Therapy Oncology Group (RTOG) [9].
Dose to PTV nodal was 45 Gy and dose prescribed to PTV primary ranged from 70 to 78 Gy. All patients received conventional protocol. Treatment plan was given in two phases.
The IMRT plans were accepted if ≥ 95% of the PTV received ≥ 95% of the determinant dose. Dynamic multi-leaf collimators were used to shape the fields. Multiple field plans were used. Eclipse version 7.0 (Varian Medical Systems, Inc, Palo Alto, Calif ) was used for all treatment planning.
Quality Assurance (QA) including QA for IMRT treatment planning, IMRT delivery system QA and patient specific QA to make sure that the delivered dose distributions agree with the planned ones. X-ray digital portal images using bone landmarks were done daily before RT session for all cases.
Androgen Deprivation Therapy (ADT)
All patients received Androgen Deprivation Therapy (ADT) using (Luteinizing Hormone Releasing Hormone) LHRH analogue with anti-androgen (in the first week) as neoadjuvant, concurrent and adjuvant. Intermediate risk group patients received short term ADT (4-6 months) while high risk group patients will continue long term ADT for 2-3 years.
Patient assessment
Toxicity data were collected from all patients during the last week of radiotherapy, 3 months and 6 months after finishing radiotherapy according to the Common Terminology Criteria for Adverse Events (CTCAE version 5.0). Assessment of quality of life done for all patients as base line, during the last week of radiotherapy, 3 months and 6 months after finishing radiotherapy by using EORTC QLQ-C30 for general health related quality of life (HRQOL) and QLQ-PR25 for prostate specific Health Related Quality of Life (HRQOL). The higher the score was, the worse the quality of life.
Statistical analysis
The used tests were Chi-square test for categorical variables, to compare between different groups and Student t-test for normally distributed quantitative variables, to compare between two studied groups by IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp).
End points
The end points of this study were evaluation of acute toxicity and assessment of quality of life for our patients. Acute toxicity was defined any reaction related to radiation that occurred up to 6 months after radiation course.
Results
The clinic pathological features of patients in both groups were gathered in (Table 1).
| 3DCRT (n=15) | IMRT (n=15) | p | |||
| No. | % | No. | % | ||
| Age (years) Mean ± SD | 67.87 ± 5.42 | 68.13 ± 7.64 | 0.913 | ||
| Smoking | |||||
| No | 12 | 80 | 9 | 60 | 0.427 |
| Yes | 3 | 20 | 6 | 40 | |
| Comorbidity | |||||
| No Comorbidity | 5 | 33.3 | 8 | 53.3 | 0.145 |
| DM | 7 | 46.7 | 2 | 13.3 | |
| HTN | 1 | 6.7 | 0 | 0 | |
| DM+HTN | 2 | 13.3 | 2 | 13.3 | |
| HTN+IHD | 0 | 0 | 2 | 13.3 | |
| Chronic osteoarthritis | 0 | 0 | 1 | 6.7 | |
| Family history | |||||
| No | 15 | 100 | 14 | 93.3 | 1 |
| Yes | 0 | 0 | 1 | 6.7 | |
| Previous pelvic surgery | |||||
| No | 14 | 93.3 | 11 | 73.3 | 0.33 |
| Yes | 1 | 6.7 | 4 | 26.7 | |
| T stage | 0.659 | ||||
| T1c | 1 | 6.7 | 2 | 13.3 | |
| T2b | 6 | 40 | 5 | 33.3 | |
| T2c | 6 | 40 | 7 | 46.7 | |
| T3a | 0 | 0 | 1 | 6.7 | |
| T4 | 2 | 13.3 | 0 | 0 | |
| Mean ± SD Initial PSA | 26.69 ± 18.58 | 28.79 ± 22.71 | 0.784 | ||
| Gleason score | 0.365 | ||||
| ≤ 6 | 0 | 0 | 5 | 33.3 | |
| 7 | 13 | 86.6 | 9 | 60 | |
| 10-Aug | 2 | 13.3 | 1 | 6.7 | |
| Risk group | 0.404 | ||||
| Intermediate | 7 | 46.7 | 6 | 40 | |
| High risk | 6 | 40 | 9 | 60 | |
| Very high risk | 2 | 13.3 | 0 | 0 | |
| Total dose Gy Mean ± SD | 73.31 ± 2.60 | 76.13 ± 1.60 | 0.002* | ||
Tab. 1. Comparison between the clinicopathological features of patients in both groups
Toxicity assessment
Gastrointestinal toxicity: During radiotherapy, 80% 0f patients in group A had G1 abdominal pain vs 46.7% in group B (p=0.058). Diarrhoea was reported in 80% of group A (33% G2-3) versus 60% of group B (6.7% G2-3). Number of patients with proctitis (G1or G2-3) in group A was double the number in IMRT group. Rectal pain (G2-3) was experienced in 40% of patients in group A versus 0% in group B (p=0.016). Three months after radiotherapy, only grade 1 GI toxicity was reported in both groups with no significant difference. Six months after radiotherapy, GIT toxicity was limited to G1 proctitis and rectal pain (26.7% and 20% in group A vs 0% and 6.7% in group B) (Table 2).
| Grade of Gastrointestinal Toxicity |
3DCRT (n=15) | IMRT (n=15) | |||||||||||||||
| 0 | 1 | 2-3 | 0 | 1 | 2-3 | p | |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||
| During | |||||||||||||||||
| Abdominal pain | 3 | 20.0 | 12 | 80.0 | 0 | 0.0 | 8 | 53.3 | 7 | 46.7 | 0 | 0.0 | 0.058 | ||||
| Diarrhea | 3 | 20.0 | 7 | 46.7 | 5 | 33.3 | 6 | 40.0 | 8 | 53.3 | 1 | 6.7 | 0.200 | ||||
| Proctitis | 5 | 33.3 | 4 | 26.7 | 6 | 40.0 | 10 | 66.7 | 2 | 13.3 | 3 | 20.0 | 0.296 | ||||
| Rectal hemorrhage | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | ||||
| Rectal pain | 5 | 33.3 | 4 | 26.7 | 6 | 40.0 | 11 | 73.3 | 4 | 26.7 | 0 | 0.0 | 0.016 | ||||
| 3 months after | |||||||||||||||||
| Abdominal pain | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 12 | 80.0 | 3 | 20.0 | 0 | 0.0 | 1.000 | ||||
| Diarrhea | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 11 | 73.3 | 4 | 26.7 | 0 | 0.0 | 0.651 | ||||
| Proctitis | 8 | 53.3 | 7 | 46.7 | 0 | 0.0 | 11 | 73.3 | 4 | 26.7 | 0 | 0.0 | 0.256 | ||||
| Rectal hemorrhage | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | ||||
| Rectal pain | 10 | 66.7 | 5 | 33.3 | 0 | 0.0 | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 0.390 | ||||
| 6 months after | |||||||||||||||||
| Abdominal pain | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | ||||
| Diarrhea | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | ||||
| Proctitis | 11 | 73.3 | 4 | 26.7 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0.100 | ||||
| Rectal hemorrhage | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | ||||
| Rectal pain | 12 | 80.0 | 3 | 20.0 | 0 | 0.0 | 14 | 93.3 | 1 | 6.7 | 0 | 0.0 | 0.598 | ||||
Tab. 2. Gastrointestinal toxicity in studied groups during the period of study
Genitourinary toxicity: During radiotherapy, the most common GU toxicities were cystitis, dysuria and hematuria in both groups. Grade 2-3 cystitis was 33.3% of patients in group A versus 20% in group B (p=0.435) (Table 3).
| Grade of Genitourinary Toxicity | 3DCRT (n=15) | IMRT (n=15) | ||||||||||||
| 0 | 1 | 2-3 | 0 | 1 | 2-3 | p | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |||
| During | ||||||||||||||
| Cystitis | 0 | 0.0 | 10 | 66.7 | 5 | 33.3 | 2 | 13.3 | 10 | 66.7 | 3 | 20.0 | 0.435 | |
| Dysuria | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 2 | 13.3 | 13 | 86.7 | 0 | 0.0 | 0.483 | |
| Hematuria | 7 | 46.7 | 8 | 53.3 | 0 | 0.0 | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 0.020 | |
| Bladder spasm | 7 | 46.7 | 4 | 26.7 | 4 | 26.7 | 11 | 73.3 | 4 | 26.7 | 0 | 0.0 | 0.126 | |
| Urine incontinence | 8 | 53.3 | 7 | 46.7 | 0 | 0.0 | 10 | 66.7 | 4 | 26.7 | 1 | 6.7 | 0.457 | |
| 3 months after | ||||||||||||||
| Cystitis | 10 | 66.7 | 5 | 33.3 | 0 | 0.0 | 8 | 53.3 | 5 | 33.3 | 2 | 13.3 | 0.605 | |
| Dysuria | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 8 | 53.3 | 7 | 46.7 | 0 | 0.0 | 0.002 | |
| Hematuria | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 14 | 93.3 | 1 | 6.7 | 0 | 0.0 | 1.000 | |
| Bladder spasm | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 14 | 93.3 | 1 | 6.7 | 0 | 0.0 | 1.000 | |
| Urine incontinence | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 10 | 66.7 | 4 | 26.7 | 1 | 6.7 | 0.399 | |
| 6 months after | ||||||||||||||
| Cystitis | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 12 | 80.0 | 3 | 20.0 | 0 | 0.0 | 0.230 | |
| Dysuria | 12 | 80.0 | 3 | 20.0 | 0 | 0.0 | 12 | 80.0 | 3 | 20.0 | 0 | 0.0 | 1.000 | |
| Hematuria | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | |
| Bladder spasm | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | – | |
| Urine incontinence | 15 | 100.0 | 0 | 0.0 | 0 | 0.0 | 13 | 86.7 | 2 | 13.3 | 0 | 0.0 | 0.483 | |
Tab. 3. Genitourinary toxicity in studied groups during the period of study
Tab. 3. Genitourinary toxicity in studied groups during the period of study
Only Grade 1 dysuria was reported in both groups (100% in group A versus 86.7% in group B) (p=0.483). Hematuria was grade 1 in both groups (53.3% in group A versus 13.3% in group B) with significant difference (p=0.020). Three months after radiotherapy, cystitis and dysuria remained the most common GU toxicity in both groups. Dysuria was 100% and 46.7% in group A, B respectively (p=0.002). Six months after radiotherapy, one third of patients had residual grade 1 cystitis or dysuria in both groups with no significant difference.
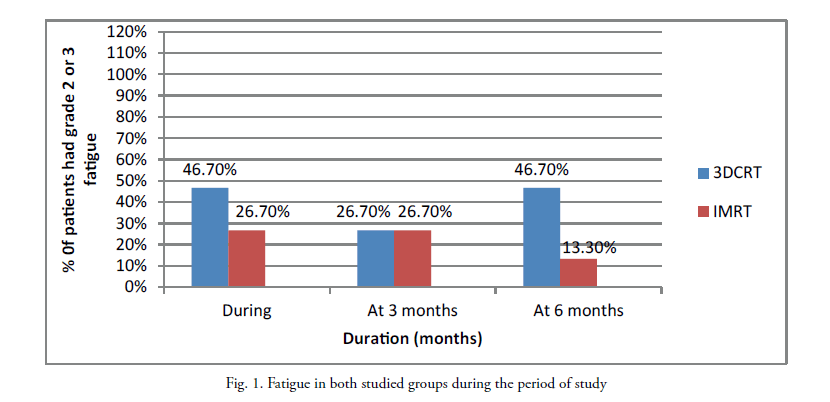
Fatigue: During radiotherapy, 46.7% of patients in group A had G2-3 fatigue versus 26.7% in group B (p=0.394). Three months after radiotherapy, G2-3 fatigue was similar in both groups. Six months after radiotherapy G2-3 fatigue was 46.7% in group A versus 13.3% in group B (p=0.195) (Figure 1).
Figure 1: Fatigue in both studied groups during the period of study
Hematological toxicity: There were no hematological events in both groups through all time points.
Quality of life assessment: Through the period of study, there was a significant difference between both groups as regard to emotional, social, role function, hormonal symptoms and sexual function which were better in IMRT group (Table 4). After 6 months the overall QoL was better in IMRT group (p=0.001).
| 3DCRT (n=15) | IMRT (n=15) | p | |
| Physical subscale | |||
| QOL0 | 7.73 ± 1.44 | 8.27 ± 3.03 | 0.545 |
| QOL3 | 7.73 ± 1.22 | 7.80 ± 3.28 | 0.942 |
| QOL6 | 7.40 ± 1.06 | 7.33 ± 2.85 | 0.933 |
| Emotional | |||
| QOL0 | 5.67 ± 0.98 | 5.40 ± 1.40 | 0.551 |
| QOL3 | 4.47 ± 0.52 | 5.80 ± 2.21 | 0.037 |
| QOL6 | 4.67 ± 0.72 | 5.47 ± 1.64 | 0.100 |
| Cognitive | |||
| QOL0 | 2.93 ± 0.70 | 2.93 ± 0.88 | 1.000 |
| QOL3 | 3.27 ± 0.70 | 2.93 ± 0.88 | 0.263 |
| QOL6 | 3.53 ± 0.52 | 3.07 ± 1.10 | 0.153 |
| Social and financial | |||
| QOL0 | 5.67 ± 1.23 | 4.27 ± 1.33 | 0.006 |
| QOL3 | 5.13 ± 1.13 | 4.0 ± 1.0 | 0.007 |
| QOL6 | 5.33 ± 0.82 | 3.47 ± 0.83 | <0.001 |
| Role function | |||
| QOL0 | 3.87 ± 0.99 | 3.53 ± 1.85 | 0.544 |
| QOL3 | 3.40 ± 0.51 | 3.0 ± 1.20 | 0.248 |
| QOL6 | 3.53 ± 0.52 | 2.47 ± 0.92 | 0.001 |
| Global | |||
| QOL0 | 6.87 ± 0.99 | 6.0 ± 2.10 | 0.165 |
| QOL3 | 5.67 ± 0.49 | 5.53 ± 1.73 | 0.777 |
| QOL6 | 5.40 ± 1.64 | 4.93 ± 1.10 | 0.368 |
| Symptoms scale | |||
| QOL0 | 18.40 ± 2.67 | 17.0 ± 3.51 | 0.229 |
| QOL3 | 15.27 ± 1.33 | 15.53 ± 3.16 | 0.767 |
| QOL6 | 15.47 ± 1.30 | 14.20 ± 2.98 | 0.143 |
| Urinary symptoms | |||
| QOL0 | 17.73 ± 3.56 | 17.93 ± 4.89 | 0.899 |
| QOL3 | 11.87 ± 1.92 | 14.0 ± 3.53 | 0.052 |
| QOL6 | 11.07 ± 1.22 | 11.27 ± 3.26 | 0.827 |
| Bowel symptoms | |||
| QOL0 | 5.53 ± 1.25 | 5.27 ± 1.62 | 0.618 |
| QOL3 | 4.73 ± 0.70 | 4.53 ± 0.52 | 0.382 |
| QOL6 | 4.33 ± 0.49 | 4.53 ± 0.52 | 0.285 |
| Hormonal symptoms | |||
| QOL0 | 8.87 ± 1.60 | 8.0 ± 1.36 | 0.121 |
| QOL3 | 10.13 ± 1.36 | 9.13 ± 1.30 | 0.049 |
| QOL6 | 10.33 ± 1.68 | 9.47 ± 1.19 | 0.113 |
| Sexual activity | |||
| QOL0 | 6.07 ± 1.10 | 5.80 ± 1.26 | 0.543 |
| QOL3 | 6.0 ± 1.07 | 5.27 ± 1.67 | 0.165 |
| QOL6 | 6.27 ± 1.22 | 5.47 ± 1.68 | 0.148 |
| Sexual function | |||
| QOL0 | 11.08 ± 1.51 | 9.67 ± 1.50 | 0.031 |
| QOL3 | 11.85 ± 1.34 | 10.0 ± 1.54 | 0.004 |
| QOL6 | 12.83 ± 1.11 | 10.0 ± 1.83 | 0.001 |
| Overall Quality of life | |||
| QOL0 | 98.20 ± 13.82 | 92.13 ± 12.77 | 0.222 |
| QOL3 | 87.93 ± 6.10 | 85.53 ± 10.43 | 0.448 |
| QOL6 | 87.60 ± 4.84 | 78.33 ± 8.86 | 0.001 |
Tab. 4. Quality of life in both studied groups during the period of study
Discussion
One of the principle definitive treatment for prostate cancer is external beam Radiation Therapy (RT). IMRT is a progress of 3D-CRT that can safely escalate the dose to non-uniform target volume by changing the intensity of the beam with potential lower radiation toxicity compared to 3DCRT [10]. In our study, cases who received definitive radiotherapy using IMRT had less sever GI toxicity but there were similar sever GU toxicity compared to 3DCRT matched with Sujenthiran et al. and Michalski et al. [10, 11]. Our results regarding incidence of rectal pain and microscopic hematuria were higher in 3DCRT group in line with RTOG 0126 prostate cancer trail, in which the use of IMRT in high dose (79.2 Gy) for men with localized prostate cancer was associated with significantly lower incidence of acute GI and GU toxicity.
Viani et al. concluded that IMRT decreased the delivery of considerable dose to bladder and rectum and this was reflected on toxicity with lower incidence of grade 2-3 GI and GU toxicity and better quality of life in IMRT [12].
Bruner et al. in RTOG 0126 prostate cancer trail, investigated and compared patient reported outcomes in similar high dose 3DCRT and IMRT and demonstrated no significant differences between IMRT and 3DCRT in bowel and urinary domains of QoL at any time point up to 24 months matched to our results [13]. Our study was a limited study done in 2 hospitals with small number of patients included in both groups so larger number of patients is required for better assessment of QoL. Also, the total radiation dose received was not constant among study population. Moreover, Self-administrated QoL questionnaires were not feasible due to lack of Arabic translated form.
Conclusion
IMRT was associated with significant lower incidence of gastrointestinal and genitourinary toxicity especially Grade 2-3. After treatment finishing QoL was better in IMRT group. IMRT for patients selected for definitive radiotherapy for prostate cancer especially in whom low dose limits for organs at risk couldn’t be achieved with 3DCRT plans. More studies in larger set of patients my possibly help in better evaluating health related quality of life in prostate cancer patients receiving definitive radiotherapy.
Conflict of Interest
The authors declare no conflict of interest.
References
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, el al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer.2018;103:356-387.
- Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, et al. Clinically localized prostate cancer: asco clinical practice guideline endorsement of an american urological association/american society for radiation oncology/society of urologic oncology guideline. J Clin Oncol.2018;36:3251-3258.
- Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer : long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464-473.
- Lilleby W, Narrang A, Tafjord G, Vlatkovic L, Russnes KM, et al. Favorable outcomes in locally advanced and node positive prostate cancer patients treated with combined pelvic IMRT and androgen deprivation therapy. Radiat Oncol. 2015;10:232.
- Latorzeff I, Mazurier J, Boutry C, Dudouet P, Richaud P. Benefit of intensity modulated and image-guided radiotherapy in prostate cancer. Cancer Radiother. 2010;14:479-487.
- Wortel RC, Incrocci L, Pos FJ, van der Heide UA, Lebesque JV, et al. Late Side effects after image guided intensity modulated radiation therapy compared to 3d-conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. Intern J Radiat Oncol. 2016;95:680-689.
- Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Intern J Radiat Oncol. 2012;84:125-129.
- Gay HA, Barthold HJ, O’Meara E, Bosch WR, El Naqa I, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas. Intern J Radiat Oncol. 2012;83:e353-e362.
- Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer:long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464-473.
- Sujenthiran A, Nossiter J, Charman SC, Parry M, Dasgupta P, et al. National population-based study comparing treatment-related toxicity in men who received intensity modulated versus 3-dimensional conformal radical radiation therapy for prostate cancer. Intern J Radiat Oncol. 2017;99:1253-1260.
- Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Intern J Radiat Oncol. 2013;87:932-938.
- Viani GA, Viana BS, Martin JEC, Rossi BT, Zuliani G. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer. 2016;122:2004-2011.
- Bruner DW, Hunt D, Michalski JM, Bosch WR, Galvin JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121:2422-2430.