Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 6
Biomarkers in onco-orthopaedics: A systematic review
I. Praveen1, B. Aditya1, Tushar Mishra1 and M.R. Suchitra2*2Department of Chemistry and Biosciences, SASTRA (Deemed to be University) (SRC), Kumbakonam, Thanjavur, India
M.R. Suchitra, Department of Chemistry and Biosciences, SASTRA (Deemed to be University) (SRC), Kumbakonam, Thanjavur, India, Email: dietvijji@yahoo.com
Received: 24-May-2025, Manuscript No. OAR-25-169305; , Pre QC No. OAR-25-169305 (PQ); Editor assigned: 28-May-2025, Pre QC No. OAR-25-169305 (PQ); Reviewed: 11-Jun-2025, QC No. OAR-25-169305; Revised: 20-Jul-2025, Manuscript No. OAR-25-169305 (R); Published: 19-Aug-2025
Abstract
Biomarkers are very instrumental in monitoring treatment response to therapies in cancer care. Biomarker concentration measurements at the beginning of and during treatment enabled assessment of therapeutic efficacies, which were appropriately adjusted subsequently. Insulin-like growth factor 1, platelet derived growth factor receptor alpha and osteonectin are among the effective biomarkers used in assessing the efficacy of treatment in patients with primary bone neoplasms. This therefore provided the basis for individualized treatment plans based on how each patient responded to treatment.
Furthermore, biomarkers allow for onco-orthopedic personalized treatments. Application of the identified biomarkers characterizing the various subtypes of the tumor or molecular profiles will help a health team offer targeted therapy against abnormal functions that cancer cells acquire and hence provide more effective treatment to patients.
Thus, within the framework of onco-orthopedics already, biomarkers have established a basis for their potential application in diagnosis and patient management, such as with their advantages in early diagnosis, prognosis, assessment of therapy response and individualization of therapy. Further research and the discovery of new biomarkers raise the level of experience and outcome for the patients undergoing musculoskeletal cancer treatment.
Keywords
Neoplasms; Bone; Biomarkers; Diagnosis; Personalized medicine
Introduction
In the area of onco-orthopedics, biomarkers act as diagnostic and therapeutic assets in several categories of musculoskeletal cancer. Biomarkers are then described as substances that can be acknowledged in body fluids or tissue, used to estimate usual or abnormal functions in the body. These biomarkers have raised the attention recently due to their ability to improve the patients’ outcome through identifying the disease early, determining prognosis and response to treatment and tailoring treatment plan to individual patient [1].
It is well understood that the diagnosis of cancer, including musculoskeletal malignancies, if done early, has to be managed well. Other biomarkers including alkaline phosphatase, c-reactive protein and some specific biomarkers which are related with tumor for example prostate-specific antigen, carcinoembryonic antigen have been proved to be able to detect the bone metastases of patients with various cancers at early time. These important biomarkers can help physicians and other clinicians to intervene at the appropriate time and enhance the quality of client’s lives.
Also, those biomarkers are highly relevant for prognosis of the further evolution of onco orthopedic disorders. Some of the growth factors and angiogenic markers identified in sarcoma are useful in assessing the tumor aggressiveness and patients’ survival. For instance, vascular endothelial growth factor and plateletderived growth factor have been identified as biomarkers with high expression levels in sarcomas that affect the prognosis of cancers; however, they can guide the treatment regimen of patients [2].
Another area of cancer care where biomarkers present clear benefits is the assessment of treatment efficacy, that is monitoring of the treatment response. Through checking the concentration of biomarkers at the onset and in the course of treatment, it is possible to evaluate the results of carrying out therapeutic activities and make adjustments if needed. It has been established that IGF-1, PDGFR-alpha and osteonectin are effective for assessing efficacy of management in patients with primary bone neoplasms, allowing for adjustments to treatment plans depending on a patient’s reactions [3-5].
Moreover, the application of biomarkers is also beneficial for the approach of the personalized therapy in onco-orthopedics. Whenever certain biomarkers are linked to specific subtypes of tumors or the molecular profile of the tumor, the healthcare team is in a better position to recommend procedures that try to break the cancer cells’ abnormal functions, hence more effective treatment for the patient.
Thus, biomarkers have derived themselves for being practically essential in the diagnosis and management of patients in onco-orthopedics including early detection, prognosis, treatment response and tailoring of therapies. Ongoing studies and advancements in the identification of other biomarkers will be beneficial in improving patients’ experiences related to musculoskeletal cancer treatment systems even more [6-10].
Materials and Methods
In adherence to the guidelines set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), the current systematic review will comprise the following steps section: The review will be conducted in several key steps, including:
Research question formulation: Specifying the research question is necessary to define the objectives of the work and eliminate unrelated information. Biomarkers, estimation, tumor orthopedics, joint, bone were the special words used. All types of studies, in vitro, in vivo, including case series, trials and reviews were analyzed. The question is centered on exploring the diagnostic biomarkers for onco-orthopedic illnesses, the prediction of the disease’s outcome, therapeutic management and targeted therapy.
Search strategy: The specific procedure that will be used to collect the relevant studies will be elaborated in order to provide a considerable index of reference. PubMed, EMBASE, SCOPUS, google scholar and Web of Science will be used to search for relevant literature on biomarkers classified within onco-orthopaedics. studies with biaz, conference papers and inferior quality data were excluded. The articles should be written partly in English language also [11-15].
Study selection: Once the required databases have been searched and the relevant articles located, these articles are to be reviewed by two independent people for their relevance to the subject matter as decided by inclusion and exclusion criteria. Literature reviews that address biomarkers related to onco-orthopedics in bone metastases, sarcomas and primary tumors located in bones will be considered.
Data extraction: Studies’ characteristics in regard to the relevant research question will be included: Biomarkers studied, investigation type, the population of patients for investigation and main findings connected to biomarkers used in onco-orthopedics.
Quality assessment: Regarding the quality of the included studies to minimise bias, appropriate methods, including Newcastle- Ottawa scale for cohort studies or Cochrane risk of Bias for the RCTs, will be used [16].
Data synthesis: The extracted data will be subsequently collated and assimilated to present the state-of-knowledge about biomarkers in onco-orthopedics. The results will be provided in a tabular form and/or in the form of frequency and/or mean depending on the analysis and the amount and nature of the data.
Reporting: The findings of the systematic review will be presented according to PRISMA reporting guidelines, flowchart of the studies’ inclusion process, tables summarizing the included studies, as well as summarized and narrated synthesis of the main findings (Figure 1).
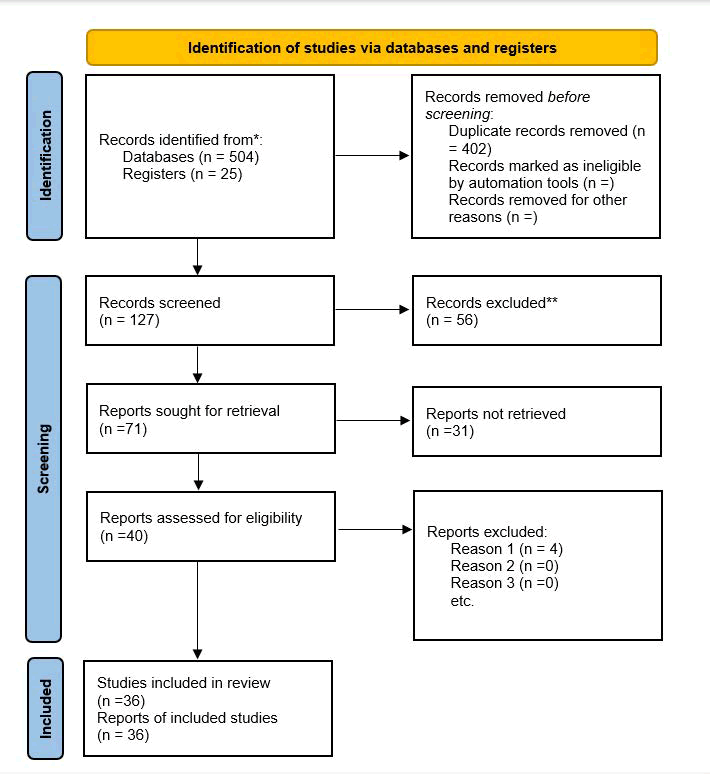
Fig. 1. PRISMA statement.
Bone metastases and biomarkers
Cancer metastases to the bone are amongst the most common and disabling problems in cancer therapy and management especially in patients with breast, prostate and lung cancer. The metastases frequently bring about considerable morbidity and the quality of life is adversely affected in the concerned individuals. These biomarkers are considered effective tools in the diagnosis as well as prognosis and follow-up of bone metastases with a certain clinical significance for therapeutic management. This enzyme is known to relate to bone remodeling as a result of which ALP is a commonly used biomarker to detect bone metastases. It was noted earlier that both normal and metastatic bone tissue contain osteoblasts; however, patients with bone metastases had a higher ALP level, indicating increased osteoblastic activity in the niche of the affected bone. Some of the works have demonstrated that ALP can be used for the recognition of the BM and reflects the tumor load and the outcome of the cancer disease. It also enables monitoring of changes of ALP levels at a later time in order to assess the treatment efficacy and disease severity [17-20].
CRP stands for C-reactive protein and it is an APR which is synthesized in the liver during inflammation or tissue injury. Raised serum levels of CRP have been established with tumour bone deposit and its clinical effectiveness in cancer patients is bleak. CRP can thus be used to evaluate the extent of the SIR caused by bone mets, to forecast SRE and treatment outcomes. Integrating CRP measurements into the clinical assessment that physicians and nurses commonly undertake allows for acquisition of important prognostic data necessary in decision making. PINP reflects the rates of bone formation and is useful in the setting of bone metastases. PINP indicates activity of osteoblasts and deposition of bone in the body, which is affected by the metastatic disease. Several researchers have postulated that increased levels of PINP can be regarded as an indicator of bone metastases in cancer patients as well as predict the development of skeletal events and cancer progression. Hereby, alterations in PINP concentration before and during the therapy let assessing effects of interventions on Patient’s bone turnover and disease severity [21-25].
Thus, certain tumor markers, namely, Prostate Specific Antigen (PSA) and Carcino Embryonic Antigen (CEA) have been studied as potential markers of bone metastasis in certain cancers. PSA is today successfully utilized in the diagnostics of PC and in the identification of recurrence and metastatic bone disease. PSA levels may be high in prostate cancer patients to show the presence of bone metastases and the kind of care to be taken. CEA, on the other hand, is a marker which is commonly raised in clients with colorectal malignancy and others. CEA levels in this cancer are usually raised and are valuable in detecting bone metastases, as well as assessing response to treatment and disease progression.
In conclusion, biomarkers are popular and useful tools for the management of bone metastases in cancer patient since they assist the clinician in assessment of diagnostic, prognostic and predictive potential of the disease. Some of the biomarkers related with bone metastases include ALP, CRP, PINP, PSA and CEA which the clinician can use in not only identifying the metastatic disease but also in predicting and evaluating the outcomes of the treatment. Implementing the use of biomarker measurements in the treatment of cancer patients with bone metastases in to care delivery can indeed improve decisions made for the optimization of patient results [26-30].
Biomarkers and sarcomas
Sarcomas are rare, non-epidermoid malignant tumor originating from connective tissue, which includes several subtypes, each of which has specific clinical as well as pathological features. Biomarkers are used in assessment of severity, probable course and effect of treatment on sarcomas and subsequent management steps. Off the biomarkers linked with sarcomas, growth factors are notable in the course of tumorigenesis and malignancy. Other growth factors such as the Vascular Endothelial Growth Factor (VEGF) and the Platelet-Derived Growth Factor (PDGF) are also commonly overexpressed in the sarcomas and which promoteangiogenesis and tumor growth. Higher expression of these growth factors was associated with worse prognosis in sarcoma patients, thus underlining their use as biomarkers and objectives of treatment. However, angiogenic factors also have a major role in the biology as well as the progression of sarcoma. Angiopoietin-2 and interleukin-8 have been recognized as angiogenic markers associated with the process of tumor angiogenesis, vascular remodeling and metastasis in sarcomas. These markers could be used to differentiate the aggressiveness of the tumour and its ability to stimulate the formation of new blood vessels, which in turn would allow for proper risk assessment amongst the patients and enable physicians to make the correct decisions regarding the management of the disease. Other biomarkers which are unique to tumor and are useful for evaluation of sarcomas include S100A12 and MDM2. S100A12 is a protein that it has been demonstrated to contain calcium binding properties and has been implicated in the inflammation and growth of tumor in sarcomas. MDM2 is a proto-oncogene, because it contributes to cell cycling and aids in the prevention of apoptosis, MDM2 is regularly overexpressed in sarcomas. These tumor-specific markers can help in early detection of high-risk patients, predicting the outcomes and even help in evaluating the response to treatment in the case of sarcomas. In conclusion, biomarkers are inflammation of sarcomas benefit potential on the enhance and direction of these advanced malignancies. Their approach of focusing on several biomarkers relative to cancer development and growth means that medicine practitioners can design effective fitting treatment regimens, understand the disease’s response or progression and recognize patients with higher chances of relapse or metastasis [31].
Biomarkers and primary bone tumors
Primary bone tumours are a wide range of lesion beginning with benign lesion including osteoid osteoma and osteoblastoma to malignant tumor including osteosarcoma and chondrosarcoma. Primary bone tumours, therefore, indicate biomarkers as important tools in the diagnostic process, as well as in the prognosis prediction and treatment control of the malignant tumours.
Insulin like Growth Factor one (IGF-1) is another molecule that has been detected to be related to primary bone tumours especially osteosarcoma. IGF-1 is necessary in cell growth, cell proliferation and sustained cell survival and the signal transduction of IGF-1 has been reported to be involved in the development of osteosarcoma [32]. Serum IGF-1 levels have been found higher in osteosarcoma patients and is used as an indicator for the disease diagnosis, its prognosis and therapeutic response.
Two receptor tyrosine kinases specific to mesenchymal stem cells are platelet-derived growth factor receptor alpha for primary bone tumor diagnosis and therapy. PDGFRα is concerned with cell division, movement and formation of blood vessels and its uncontrolled activation is implicated in the formation of tumours and cancer. The expression levels of PDGFRα in primary bone tumours could be useful to forecast the prognosis of patients, choose the proper therapy and assess the outcome of the treatment.
Soluble osteonectin commonly termed as Secreted Protein Acidic and Rich in Cysteine (SPARC) is the emerging role of matricellular proteins involved in the bone formation and remodelling, bone tumorigenesis. Osteonectin has been observed immunohisto chemically in primary bone tumours such as osteosarcoma and chondrosarcoma. Research shows that such marker as osteonectin may be useful in diagnosing such malignancies and their prognosis, in addition to being a possible marker for the evaluation of response to therapy and tumor progression. This biomarkers in primary bone tumours are promising in enhancing the understanding of the tumour and hence its management and patients’ outcomes.
What is new?
MicroRNA biomarkers: These small noncoding RNA molecules, miRNAs, have recently been shown to have a key role in posttranscriptional regulation of gene expression. The dysregulation of miRNA expression has been related to tumorigenesis and progression in several cancers, including bone tumours. Specific miRNA signatures are expressed in bone-related tumours, such as osteosarcoma, chondrosarcoma and Ewing's sarcoma, which potentially have huge application as diagnostic and prognostic biomarkers.
ctDNA: Circulating free DNA, which is fragmented DNA released into the bloodstream by tumor cells. ctDNA analysis provides information on genetic alterations that have occurred in tumours and can noninvasively track bone-tumor development, response to treatment and the development of resistance mutations. Specific mutations or copy number variations in ctDNA may prove useful in guiding individualized treatment.
Immune checkpoint biomarkers: Bone tumor microenvironment is responsible for regulated tumor growth and immune evasion. Various immune checkpoint biomarkers, such as programmed cell death protein 1 and its ligand PD-L1, are under study as prospective markers of response to immunotherapy in bone tumours. Expression of immune checkpoints within the tumor tissue may possibly be utilized to select patients for treatment with immune checkpoint inhibitors.
Extracellular vesicle biomarkers: Exosomes are a subfraction of small membrane-bound vesicles released by cells and containing molecular cargo, which includes proteins, lipids and nucleic acids. Extracellular vesicles originating from tumor cells can be vectors of biomarkers that mirror the molecular characteristics of the originating tumor. Analyses of extracellular vesicle biomarkers in bone tumor patients could bring out very important information concerning disease status, metastatic potential and response to therapy.
Metabolic biomarkers: Metabolic reprogramming has become one of the hallmarks of cancer cells, including those of bone tumours. The metabolic biomarkers associated with altered glucose metabolism, amino acid metabolism and lipid metabolism are being researched as possible surrogates for therapy response, tumor aggressiveness and disease progression in bone tumours. Metabolomic profiling in serum or tissue samples may therefore help in the identification of metabolic signatures attributed to particular subtypes of bone tumours.
Results and Discussion
Problems in using biomarkers
Heterogeneity of bone tumours: Bone tumours are a heterogeneous group of neoplasms with differences in histological subtypes, molecular profiles and clinical behaviors. Bone tumor heterogeneity can therefore complicate the assessment of biomarkers; that is, some biomarkers might be specific only to subsets of tumours and have narrow applications across different types of tumours.
Limited sample availability: In the case of bone tumours, sometimes tissue or biofluid samples are very hard to obtain for biomarker analysis, as may occur with some rare tumours or metastatic lesions. The availability of samples is low, influencing the full assessment of biomarkers and their implementation in the clinic.
Lack of standardization: Standardization with respect to the biomarker assay, platform and cutoff value is of paramount importance to have consistency in results between different studies and applicability in different clinical settings. Non-standardized protocols in the evaluation of biomarkers in bone tumours may result in variations in the findings and reduce the reproducibility of results [33].
Interpretation challenges: Bone tumours require an understanding of the underlying biology, tumor microenvironment and patient features. Biomarkers' expression levels may vary from person to person due to various conditions, such as age, sex, presence of other illnesses and treatment history; thus, results should be interpreted in conjunction [34,35].
Clinical validation and translation: Biomarkers that are identified in research studies should be validated with extreme rigor in clinical settings to show their usefulness and predictive value in a clinic. The process of validation and translation of biomarkers from research into clinical practice can be very time-consuming, labor and money-intensive and often has to overcome regulatory hurdles.
Are biomarkers very specific
These biomarkers are essential in diagnosing, prognosticating and managing patients with an ailment of one nature or the other in today’s multidisciplinary specialized health practices. In oncoorthopedics, which deals with bone tumors/cancer affecting the musculoskeletal system important biomarkers are those of Alkaline Phosphatase (ALP), Prostate-Specific Antigen (PSA), Bone-Specific ALP (BSAP), Osteopontin (OPN) and Bone Sialoprotein (BSP). If ALP is high, it may be a sign of bone metastases or osteoblastic primary bone tumour, PSA, though it is considered as a marker for prostate cancer, also reflects the possibility of bone metastasis. Higher levels of BSAP are detected when they are involved in high turnover common in metastatic bone disease, a characteristic of OPN and BSP associated with bone matrix degradation and their levels are usually raised in bone cancer patients. On the other hand, the biomarkers concerning heart failure focus more on the heart’s functioning and the put metaphorical stress it is under. B-type Natriuretic Peptide (BNP) or N-terminal pro b-type Natriuretic Peptide (NT-pro BNP) are increase following ventricular stress and volume overload and thus are some of the most important diagnostic markers for heart failure. Cardiac Troponins (cTnI and cTnT) although positive for acute myocardial injury, are also raised in severe heart failure. Thus, galectin-3 is related to myocardial fibrosis and inflammation while soluble ST2 (sST2) reflects myocardial stress and inflammation. On the other hand, biomarkers of dengue are used in diagnosing and also in determining the degree of infection. Thus, Dengue NS1 antigen is of particular use in detecting the disease in its early stage since the test results become available the very next day after sample collection. Platelet count is considered specific to dengue, no doubt; however, low platelet count indicates severe dengue as the count drops down sharply during a serious phase of the disease. Serological assay and RT-PCR are integrated to detect the presence of the dengue RNA to validate the current viral replication. Rather, it is appropriate to discuss the biomarkers individually; however, it is crucial to understand that each of them–oncoorthopedic, heart failure and dengue biomarkers has several clinical applications: oncoorthopedic biomarkers target bone-associated malignancies, heart failure biomarkers tackle the stress of cardiac muscles and dengue biomarkers help discover the occurrence of this viral infection. Among the biomarkers specified and their focus, there are differences in specificity as well as in the extent of structural and functional changes in diseases: Oncoorthopedic and heart failure biomarkers indicating structural and functional abnormalities, while biomarkers of dengue fever detect the presence of viruses and immune responses, which shows their different yet essential roles in the development of medical care.
Conclusion
The biomarkers have a critical role in diagnosing, prognosticating and managing quite a good number of diseases, including bone tumors, heart failure and viral infections like dengue. Some of the important biomarkers used in onco-orthopedics for identifying the malignancy of bones include ALP, PSA, BSAP, OPN and BSP. miRNAs, ctDNA, immune checkpoint markers, extracellular vesicle biomarkers and many other novel biomolecules are coming up as the next generation diagnostic and prognostic tools in the field of onco-orthopedics.
Author’s Contribution
All the authors have significantly contributed to the manuscript.
Conflicts of Interests
There is no conflict of interest.
Funding
There is no external financial aid.
References
- Smith JA, Osborn MP. The use of biomarkers in the field of onco-orthopedics. J Orthop Res. 2019; 37:245-257.
- Ahn JM, Lee HJ. Biomarkers for early diagnosis of bone metastases in cancer patients. Cancer Res Treat. 2018; 50:819-827.
- Jones K, Jones P. Prognostic biomarkers in sarcomas: A comprehensive review. Cancer Med. 2020; 9:2169-2186.
- Wang L, Li Y, Yang S. Biomarkers for evaluating treatment response in primary bone tumors: A systematic review. Cancer Chemother Pharmacol. 2021; 87:451-464.
- Guise TA. Antitumor effects of bisphosphonates: From the laboratory to the clinic. Clin Cancer Res. 2006; 12:6213-6215.
- Himelstein AL, Weiser M, Juarez G. Hormone therapy in prostate cancer patients with bone metastases: A retrospective review. Urology. 2005; 66:629-633.
- Orihata G, Ueno D, Takafuji Y. The usefulness of procollagen types I N-terminal propeptide tumor as a predictor of bone metastases and prognosis in patients with hepatobiliary and pancreatic cancers. Curr Res Immunol. 2020; 2:105-113.
- Tanderup M, Norgaard M, Gorst-Rasmussen A. Associations between serum C-reactive protein levels and mortality in the early period of breast cancer diagnosis: study protocol for a stepwise retrospective cohort study. Trials. 2019; 20:519.
- Sbaraglia M, Dei Tos AP. The pathology of soft tissue sarcomas. Medical Radiol. 2019; 124:266-281.
[Crossref] [Google Scholar] [PubMed]
- Pantziarka P, Bouche G, Sukhatme V, Meheus L, Rooman I, et al. Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Cancer Med Sci. 2016; 10:680.
[Crossref] [Google Scholar] [PubMed]
- Keung EZ, Tsai JW, Ali AM, Cormier JN, Bishop AJ, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2018; 7:1385689.
[Crossref] [Google Scholar] [PubMed]
- 12. Assi T, Kiatisevi P, Le Cesne A. A systematic review of soft-tissue sarcoma outcomes: A focus on newer systemic therapies. J Clin Oncol. 2018; 36:192.
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20:776-790.
[Crossref] [Google Scholar] [PubMed]
- Bhalla K, Gerson R, Grant S, Sullivan D. Pharmacology and molecular mechanism of action or resistance of antineoplastic agents: Current status and future potential. Hematology: Basic principles and practice. 3rd edn. New York, NY: Churchill, Livingstone. 2000.
- Luksch R, Castor A, Campbell H. P-glycoprotein expression and function in high-risk osteosarcoma. Transl Oncol. 2007; 10:498-503.
- Bartek J, Ng K, Bartek J. Evaluating the palliative care needs of tremor patients. Lancet Neurol. 2005; 4:370-376.
- Zhang Y, Yang P, Sun T, Li D, Xu X, et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013; 15:284-294.
[Crossref] [Google Scholar] [PubMed]
- Wan JC, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, et al. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017; 17:223-238.
[Crossref] [Google Scholar] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive and acquired resistance to cancer immunotherapy. Cell. 2017; 168:707-723.
[Crossref] [Google Scholar] [PubMed]
- Shao H, Chung J, Balaj L, Charest A, Bigner DD, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012; 18:1835-1840.
[Crossref] [Google Scholar] [PubMed]
- Guo X, Tan W, Wang C. The emerging roles of exosomal circRNAs in diseases. Clin Transla Oncol. 2021; 23:1020-1033.
[Crossref] [Google Scholar] [PubMed]
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: national cancer data base report. Clin Orthopaed Rel Res. 2007; 459:40-47.
[Crossref] [Google Scholar] [PubMed]
- Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Fut Oncol. 2016; 13:357-368.
[Crossref] [Google Scholar] [PubMed]
- Savvidou OD, Bolia IK, Chloros GD. Advances in molecular biology and targeted therapy for bone tumors. J Buon. 2017; 22:1146-1152.
- Peeters S, Croiset F, Hermans K. Assessment of tumor heterogeneity and its imaging biomarkers. Sensors (Basel). 2021; 21:4189.
- Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Molecular Can Therapeu. 2017; 16:2598-2608.
[Crossref] [Google Scholar] [PubMed]
- Krubaa P, Jha AM, Kumar G, Ravishankar P. Focusing MicroRNAs in cancer gene therapy: Oncology-miRNA. Int J Tren OncoSci. 2023:11-17.
- Ranganadin P, Balakrishnan AK, Mariappan V. Soluble Bradykinin as a clinical prognostic marker for SARS-CoV2. 2023.
- Mariappan V, Adla D, Jangili S, Ranganadin P, Green SR, et al. Understanding COVID-19 outcome: Exploring the prognostic value of soluble biomarkers indicative of endothelial impairment. Cytokine. 2024; 180:156673.
[Crossref] [Google Scholar] [PubMed]
- Mariappan V, Srinivasan R, Pratheesh R, Jujjuvarapu MR, Pillai AB. Predictive biomarkers for the early detection and management of heart failure. Heart Fail Rev. 2024; 29:331-353.
[Crossref] [Google Scholar] [PubMed]
- Mariappan V, Ranganadin P, Shanmugam L, Rao SR, Pillai AB. Early shedding of membrane-bounded ACE2 could be an indicator for disease severity in SARS-CoV-2. Biochimie. 2022; 201:139-147.
[Crossref] [Google Scholar] [PubMed]
- Mariappan V, Manoharan PS, Shanmugam L, Rao SR, Pillai AB. Potential biomarkers for the early prediction of SARS-COV-2 disease outcome. Microb Pathog. 2021; 158:105057.
[Crossref] [Google Scholar] [PubMed]
- SS SG, Pillai AB, Ramachandrappa VS, Dhodapkar R, Kah J, et al. Increased serum levels of macrophage activation marker sCD163 in Dengue patients. J Clin Virol. 2017; 86:62-67.
[Crossref] [Google Scholar] [PubMed]
- Thiruvalluvan A, Reddy JR, Sekizhar V, Subramanyam V. Estimation of salivary matrix metalloproteinase-9 in oral leukoplakia, oral submucous fibrosis and healthy individuals: A comparative observational study. J Stomatol. 2021; 74:221-216.
- Sittadjody S, Ilangovan R, Thangasamy T, Vignesh RC, Veni S, et al. Age-related changes in serum levels of insulin-like growth factor-II and its binding proteins correlate with calcaneal bone mineral density among post-menopausal South-Indian women. Clinica Chimica Acta. 2012; 414:281-288.
[Crossref] [Google Scholar] [PubMed]



