Original Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 1
A propsective oncotherapeutics: Isolation, partial purification and assessment of anti-coagulant properties of Tridax procumbens from leaf extracts
Ramesh Bandla1,2,4, Madhava Krishna4, Jaisatya Gowri Gogada1, Navya Anthagiri4, Praveen Boddana33, Sugunakar Vure1, Abdul Qadeer Mohammed4, Rajesh Medisetty4 and Raghu Gogada1,2*2Department of Analytical R & D Division, Biological E. Limited, Hyderabad, Telangana, India
3Department of Plant Pathology, M.S. Swaminathan School of Agriculture (MSSSoA), Centurion University of Technology and Management (CUTM), Parlakhemundi-761211, Odisha, India
4Department of Biochemistry and Plant Physiology, M.S. Swaminathan School of Agriculture (MSSSoA), Centurion University of Technology and Management (CUTM), Parlakhemundi, Odisha, India
Raghu Gogada, Department of Biochemistry and Plant Physiology, M.S. Swaminathan School of Agriculture (MSSSoA), Centurion University of Technology and Management (CUTM), Parlakhemundi, Odisha, India, Email: rgsl@uohyd.ac.in
Received: 02-Jan-2025, Manuscript No. OAR-25-161077; , Pre QC No. OAR-25-161077; Editor assigned: 07-Jan-2025, Pre QC No. OAR-25-161077; Reviewed: 21-Jan-2025, QC No. OAR-25-161077; Revised: 01-May-2025, Manuscript No. OAR-25-161077; Published: 29-May-2025
Abstract
T Medicinal plants, mostly herbs have been widely used since ancient times for the treatment of various human diseases including cancers, with the advent of technology and extensive research relating use of herbal drugs in the health care sector has increased. The WHO reports, almost 80% of people serve through medicinal plants and their products. About 25% of active pharmaceutical drugs were derived from herbal plants. This study investigates the potential anticoagulant properties of Tridax procumbens, a medicinal plant. The study demonstrates the isolation procedure of partially purified leaf extract from the plant Tridax procumbens The active fractions from the plant material were isolated and partially purified and the anticoagulant activity of these fractions was assessed through in vitro assays like clot-based assays, APTT, HEPTEST and chromogenic assays. We elucidate the impact of extracts from Tridax procumbens on coagulation parameters. Our finding reveals the anticoagulant properties of the leaf extracts are better than aerial parts of the plant. Hence, crude leaf extracts were purified. The average relative potency for anti-factor Xa of purified leaf extract by clot-based and chromogenic assays was 42 and 31 IU/mL respectively. The clotting times by APTT and HEPTEST for the purified leaf extracts ranged between 37 to 78 seconds for concentrations of 1 to 5 IU/mL and 9 to 30 seconds for concentrations of 0.6 to 9.6 IU/mL. Further investigation on the purification of leaf extracts to high purity, their underlying mechanism and safety profiles is warranted. This paper provides the preliminary findings and further extensive research is required to assess the feasibility of incorporating the purified leaf extracts in anticoagulation therapies.
Keywords
Medicinal plant; Herbal drugs; Blood coagulation; Hemostasis; Secondary metabolites; Extraction
Introduction
Cardiovascular diseases remain a leading cause of global morbidity and mortality necessitating continuous exploration of novel therapeutics. Natural products, particularly plant extracts, have emerged as valuable reservoirs of bioactive compounds with potential anticoagulant properties. Numerous plants have been investigated for their anticoagulant potential, revealing a plethora of bioactive compounds such as flavonoids, tannins, alkaloids and polyphenols (Akram and Rashid). Each of these compounds exhibits distinct interaction with the coagulation factors, platelets and endothelial cells influencing the overall hemostatic balance. Several medicinal plant preparations have shown disruption of the coagulation cascade which was confirmed by variations in clotting time [1].
Tridax procumbens, usually known as coat buttons or tridax daisy, is a type of blooming plant from the family Asteraceae. It is the most popular weed and nuisance plant that is local to the tropical Americas including Mexico, yet, it has been acquainted with tropical, subtropical and gentle mild regions around the world. Tridax procumbens is a perennial herb that has a creeping stem that can reach from 8-30 inches (20 cm-75 cm) long [2]. The leaves of Tridax procumbens are opposite, pinnate, oblong to ovate and 1-2 inches (2.5 cm-5 cm) long with cuneate bases, coarsely serrate margins and acute apexes. Its fruit is a hard achene covered with stiff hairs and has a feathery, plume-like white pappus at one end. Tridax procumbens L. is also considered a medicinal plant and recent studies have shown a growing interest in Tridax procumbens due to its diverse bioactive constituents. It is used as a drink to treat bronchial catarrh, diarrhea, dysentery, wound healing, hyperuricemia, oxidative stress, bacterial infection and liver diseases. Particularly, the exploration of its anticoagulant potential, given the pivotal role of coagulation in cardiovascular disorders. While several synthetic anticoagulants exist, the quest for safer and more sustainable alternatives drives the investigation into natural sources [3].
In this study, we have attempted to extract and partially purify the bioactive component that showed anticoagulant properties. The activity of the partially purified fraction was also evaluated by aPTT. HEPTEST, cot-based assay and chromogenic assay.
Materials and Methods
Cuvettes used for APTT and Heptest were from Stago (Catalogue No: 38876) and Human fresh plasma was from the red cross blood Bank. Control plasma was from Stago (Catalogue No: 00621), STA-0.025 M and CaCl2 for APTT was from Stago (Catalogue No: 00367). Kits for STA CLOT® Heparin â (Ref 00691) and C.K. Prest® â¡ (Ref 00598) were from Stago for HEPTEST and aPTT respectively [4].
Human anti-thrombin-III 10 IU/vial (Catalogue No: 82072039), Bovine factor Xa-71 nkat/vial (Catalogue No: 82098539) chromogenic substrate for factor IIa (Catalogue No: S2238). The chromogenic substrate for factor Xa (Catalogue No: S2765) was from Chromogenix. Human thrombin -10 or 100 NIH/vial (factor IIa-Catalogue no: EZ0060) was from hyphen biomed. USP Enoxaparin sodium for bioassays (Catalogue No: 1235831; Lot No: F0K265) was used as a reference standard in clot-based assay and potency by chromogenic assay and the same was used in aPTT and HEPTEST for comparison of coagulation times. Lovenox® (reference listed drug, Lot No: BRLF016) was used as a test sample for the assessment of potency by clot-based assay and chromogenic method. Source Q15 fast flow resin was from Cytiva®, catalog number 17094703. All the chemicals, reagents and solvents were of analytical grade [5].
Collection of plant material
The healthy aerial parts of the plant (stem, leaves and flowering body) and fresh leaves of Tridax procumbens were collected from Shameerpet, Hyderabad. The aerial parts of the plant and leaves were collected separately and they were washed thoroughly with running tap water in a sieve to remove the dirt and soil from the plant material. The material was soaked in 15 liters of Milli Q water to remove large waste that was not removed by a sieve filter. The material was removed from the water and the excess water was removed by blotting with dry cotton cloth and later with blotting paper [6].
Preparation of plant extract
The plant material was dried in the shade for 17 to 20 days. The dried plant materials were crushed to powder using a grinder (household blender). The dry powder of the aerial parts of the plant and leaves materials were kept in the dry bottle until further use.
These powders were subjected to different extraction methods like solvent extraction, maceration and steam distillation based on the specific characteristics of the bioactive compounds sought. However, the soxhlet extraction procedure was finalized [7].
Soxhlet extraction procedure
About 100 grams of dry whole plant and leaves powder were taken and soaked in 500 mL of water as aqueous extract and 500 mL of mixture of acetone and n-hexane solvent (50:50) as organic extract. The extractions were performed using a soxhlet extractor for 24 hours and resulting extracts were concentrated to 50 mL using rotavapor. The concentrated material was stored at 2°C-8°C until further use [8].
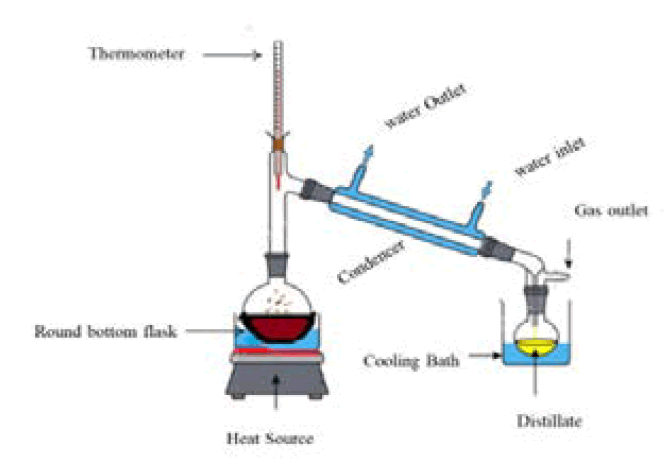
Briefly, the sample powder of the aerial part of the plant and leaf extract power was placed into the thimble and ensured that the samples were securely packed within the thimble to prevent loss during the extraction. Placed the loaded thimble into the main chamber of the soxhlet extractor. Ensured that the thimble fits snugly into the extractor to prevent any leaks or spills during the extraction process. Added the solvent, typically 250 mL, to a round bottom flask. Placed the flask onto a heating mantle to heat the solvent. Attached is the soxhlet extractor above the round bottom flask. Ensured that the extractor is securely attached to the flask to prevent any movement during the extraction process. Attached is a reflux condenser above the extractor. Positioned the condenser so that cold water enters at the bottom and exits above. This setup allows for the efficient condensation of solvent vapors, preventing solvent loss during the extraction process. Heat the solvent to reflux using the heating mantle. Reflux is achieved when the solvent vaporizes and rises into the extractor, where it extracts the desired compounds from the sample material. The condensed solvent then drips back into the flask, completing the extraction cycle (Figure 1). The resulting extracts were concentrated to 50 mL using rotavapor. The concentrated material was stored at 2°C-8°C until further use [9].
Fig.1. A representative pictogram of the soxhlet extractor, a continuous hot percolation process.
Processing of plant extracts
The soxhlet extraction fractions of the aerial parts of the herb and leaves were brownish green and dark green respectively. The depigmentation of the extracts was performed by passing the concentrates in the ratio of 1:1 with 80% acetone (50:50) through activated charcoal. The obtained filtrate was further passed through ChloroFiltr® and the resulting extracts were stored at 2°C-8°C until further use [10-12].
Purification of crude plant extract
The crude leaf extracts were purified using Fast Protein Liquid Chromatography (FPLC) with a resin source Q15 fast flow (Strong Anion Exchange Chromatography (SAXC)). The columns used for separation was FINELINE Pilot 35 with a column volume of 140 mL. A linear gradient of 0.01 to 2.5 M NaCl was used to purify the extracts. The method conditions used for the separation are detailed in Supplementary Data (SD) Table 1. The resin was regenerated using 2.5 M NaCl. The fractions of different peaks were collected and stored at 2°C-8°C until further use [13].
Clot based assay
The relative potency of the extracts obtained from the aerial parts of the plant and leaves was tested by comparing the concentration necessary to prevent the clotting of sheep plasma. The reference standard of enoxaparin sodium for bioassays (USP Catalogue No: 1235831, Lot No: F0K265) was used as the reference standard and Reference Listed Drug (RLD) Lovenox® was used as a test control sample. The relative potency of clot based method was performed as per the IP monograph for Heparin Sodium (2014), a parent molecule for enoxaparin sodium. However, enoxaparin sodium is used as a reference standard instead of heparin sodium. Briefly, citrated blood collected from the sheep (1 volume of 8% w/v% sodium citrate: 19 volumes of collected blood) was centrifuged and the resulting plasma was used. The response of the resulting plasma was considered qualified for testing the potency when the addition of 1 mL of plasma to 200 μL of 1% w/v solution of CaCl2 solution resulted in the formation of a prominent clot. The standard solution, Enoxaparin sodium solution and test sample, plant extracts were diluted to 1IU/mL for anti-factor Xa and distributed to 15 tubes as detailed in Supplementary Data (SD) Table 2. The tubes were allowed to stand for 1 hour. The clot formation was observed based on the grade of 0.25 (fluid), 0.50 (semi-clot) and 0.75 (full clot). Calculate the potency using the paired averages method as detailed in the IP monograph for heparin sodium [14-16].
HEPTEST
HEPTEST is used for quantitative determination of the potentiating effect of Unfractionated Heparins (UFH) and of Low Molecular Weight Heparins (LMWH) on anti-thrombin by measuring in plasma the anti-Xa activity as applied in a clotting method. The kit from STA® STACLOT® HEPARINâ from Stago was used for HEPTEST analysis. Briefly, the assays were performed at 37°C. Before testing, a convenient volume of reconstituted reagent 1 (substrate plasma of human origin, lyophilized) and reagent 2 (~0.3 IU of bovine factor Xa per ml of reagent after reconstitution) was warmed at room temperature for at least 60 minutes. Placed the reconstituted reagent 3 (lyophilized 35 mM phospholipids in calcium-containing medium) in the reagent socket of the instrument at 37°C. Plasma containing 25 μL of purified leaf extract and USP RS enoxaparin sodium having concentrations 0.6,1.2, 2.4, 4.8 and 9.6 IU/mL was added to cuvettes stationed on a coagulometer. To this, 50 μL of reconstituted reagent 1 is added and incubated for 1 minute followed by the addition of 50 μL of reagent 2. After 1 minute of incubation, reagent 3 was added and mixed well. The coagulation times were noted with the help of magnetic beads in the vials. Whenever a clot starts to form the magnetic beads stop due to resistance in the movement and the instrument identifies that, as the clot formation time [17].
Activated Partial Thromboplastin Time (aPTT)
The frozen human plasma obtained from the blood bank was brought to room temperature and 2 mL aliquots were prepared and frozen until further use. The 2 mL stored fractions were used for the study and discarded as biohazard upon a single freezethaw. The aPTT analysis is performed by C.K. Prest® kit for the determination of the kaolin-activated Partial Thromboplastin Time (aPTT) according to Langdell R.D, et al. and Larrieu M.-J., Weilland C. Briefly, the 0.05 mL of undiluted Human plasma or control plasma was added to 0.05 mL of premixed reagent (mixture of reagent 1 and 2) provided in the kit and 0.05 mL different concentration of extracts obtained from Purified Leaf Extract (PLE) in the concentration range of 0 5 IU/mL-1.5 IU/mL and were mixed to homogeneity. The resulting solution was incubated for 3 min at 37°C and the coagulation times were noted with the help of magnetic beads [18].
Chromogenic assay
The method for determination of potency for the purified leaf extracts was adapted from the USP monograph for enoxaparin sodium (USP 208). The active moiety present in the purified leaf extracts binds with anti-thrombin III was assumed to inhibit the activity of factor Xa and Factor IIa. The leftover uninhibited factor Xa or factor IIa cleaves the chromogenic substrates resulting in the colour development that can be monitored at 405 nm. Briefly, three buffers were prepared as follows pH-7.4 buffer (50 mM Tris with 150 mM NaCl), pH 7.4 PEG buffer (50 mM Tris, 150 mM NaCl with 0.1% w/v PEG 6000) and pH 8.4 buffer (50 mM Tris, 175 mM NaCl and 7.5 mM EDTA) [19-22].
Dilution of the test sample and reference standard: The USP Enoxaparin sodium for bioassays (Catalogue No: 1235831; Lot No: F0K265) and Lovenox® (RLD) were serially diluted using pH 7.4 buffer from 100 IU/mL to 0.2, 0.1, 0.05, 0.025 IU/mL (S4, S3, S2, S1). The test sample of purified leaf extract whose potency was known through clot-based assay was used to serially dilute using pH 7.4 buffer to get the concentrations of 0.2, 0.1, 0.05, 0.025 IU/mL (T4, T3, T2, T1) [23-27].
Preparation of reagents: Reagents were diluted for anti-factor Xa and IIa as per the tables represented in the Supplementary Data (SD) Tables 3 and 4 respectively.
Procedure: In a 96-well nunc microplate using a multi-channel pipette, 50 μL of pH 8.4 PEG buffer was added to blank wells (B1, B2, B3 and B4). Added 50 μL each of standards (S1, S2, S3 and S4) in duplicates and test samples (T1, T2, T3 and T4) in duplicates to any two continuous rows in a 96-well plate. Placed the 96 well-plate containing blank, standard and test samples in a thermoshaker and incubated at 37°C and 700 RPM for 10 minutes. Added 100 μL of Antithrombin III solution, using a multichannel pipette, mixed and incubated for 1 min at 37°C and 700 RPM. Added 25 μL of Bovine factor Xa/thrombin solution and incubated for 1 min at 37°C and 700 RPM. Added 50 μL of Chromogenic Antifactor Xa/thrombin substrate solution using multichannel pipettes, mixed well and incubated for 4 min at 37°C and 700 RPM. To stop the reaction, add stop solution and read the plate at the absorbance at 405 nm [28-32].
System suitability: The following system suitability was considered for the assay to be pass.
The %RSD between the four blanks shall not be more than 5. The parallel-line assay suitability tests for the significance of (i) Regression, (ii) Linearity and (iii) Parallelism must pass.
Data analysis: When system suitability criteria for the blank readings were met. Determined the relative potency by parallel line analysis for anti-FXa and/or anti-FIIa using Parallel Line Analysis (PLA 3.0) software. The precision of the method was calculated for six independent preparations. The ratio was reported by the ratio of determined anti-factor Xa and IIa respectively (USP 208) [33].
Statistical analysis: Results are presented as mean ± Standard Deviation (SD) for a sample size of n=6. Student’s t-test was used to determine the level of significance (p and lt;0.05). The data for precision was represented in % Relative Standard Deviation (%RSD) [34,35].
Results and Discussion
The aerial parts of the plant and the leaves were independently treated with different extraction methods like solvent extraction, maceration and steam distillation. Steam distillation and maceration crude extracts did not yield any anticoagulation activity by aPTT (data not shown). However, solvent extraction by Soxhlet extraction using a mixture of Acetone and n-hexane solvent (50:50) as organic extract yielded mild anticoagulant activity by aPTT. The sulfated polysaccharides isolated by Naqash and Nazeer showed prolonged anticoagulant activity for the time of 113 seconds by aPTT which was four times the control sample [36].
The possibility that other techniques like maceration and steam distillation did not work because the bioactive substance may not be a volatile component of the crude extract or the extended heating during the extraction process can often result in the degradation of compounds present in the sample. This phenomenon occurs due to prolonged exposure to high temperatures leading to chemical alteration or breakdown of the desired compounds.
Hence, Soxhlet extraction using organic solvents was finalized as the method of extraction. When extracts of aerial parts of the plant were compared to leaves, leaf extract showed better anticoagulant activity. Therefore, attempts were made to purify the bioactive compound in leaf extract using advanced chromatographic techniques. The crude extracts were pigmented, to remove the pigmentation, adsorption through activated charcoal following 80% acetone wash led to the removal of pigments. An additional step of passing these extracts through ChloroFiltr® removed the remnant color from the extract. These depigmented crude leaf extracts were partially purified using strong anion exchange chromatography [37-40].
The study conducted by Durgacharan, et al., has shown the aqueous leaf extracts have antidiabetic activity experimental studies revealed that the aqueous and alcoholic extracts from Tridax procumbens leaf (200 mg/kg) orally administered for 7 days produced a significant decrease in the blood glucose level in the model of alloxan-induced diabetes in rats. Other investigators have also assessed the hypoglycemic response for the extracts obtained from Tridax. The zinc nanoparticles and leaf extracts showed a significant hypoglycemic response in a dose-dependent manner [41-43].
The extracts of Tridax procumbens were shown to have antimicrobial and anti-oxidant activity. Several other investigators also studied its wound-healing properties. There are also reports discussing its role in osteoclast formation, anti-inflammatory and bone regeneration [44].
The aerial parts of the plant and leaves were extracted in aqueous and organic phases. The aqueous extract did not show prominent coagulation activity by aPTT (data not shown). However, the organic extract of Acetone and n-Hexane (50:50) showed anticoagulant activity. Hence further investigation was performed on the organic extract.
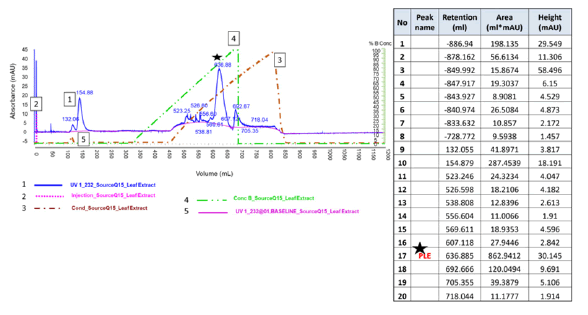
The crude organic extracts of the aerial parts of the plant and the leaves were assessed for potency by clot-based assay. The average potency for the crude extracts of the aerial parts and the leaves was 11 ± 1.47 and 27 ± 2.80 respectively. The potency for the crude extracts of the aerial parts and the leaves ranged from 9 to 13 IU/mL and 23 to 30 IU/mL respectively. Further, the potency of the leaf extract improved upon Ion exchange purification. The potency of the purified fractions ranged between 38 to 47 IU/ mL. The average potency of the crude extracts of leaves was better than the aerial parts of the plant. Hence, leaf extract was further purified using the Source Q15 Fast Flow resin (conditions used for purification are detailed in the methods section). The crude leaf extracts upon linear gradient elution of 20 mM Tris with 0.01NaCl to 2.5 NaCl at pH=8.00 lead to various peaks, from the cluster of the peaks, elution fraction at Retention Time (RT) 636.88 minutes showed anticoagulant activity of 42 (± 9) IU/mL (Figure 2). It is evident from the clot-based assay that the potency of the partially Purified Leaf Extract (PLE) fraction was 3.8 times higher than the crude aerial plant (Table 1). Hence, other coagulation tests were performed on the PLE fractions. The PLE fractions were subjected to GC-MS analysis (data not shown), which yielded the major moiety of Azulene. This fraction was used to assess the anticoagulation activity [45,46].
Fig.2. Chromatographic profile for purification of the Purified Leaf Extract (PLE). The star symbol in the chromatograph represents the fraction of interest.
| S. no | Estimated potency (IU/mL) | ||
|---|---|---|---|
| Crude aerial plant extract | Crude leaf extract | Purified fraction of leaf extract | |
| 1 | 12 | 28 | 38 |
| 2 | 10 | 26 | 40 |
| 3 | 9 | 30 | 47 |
| 4 | 13 | 24 | 45 |
| 5 | 11 | 29 | 43 |
| 6 | 10 | 23 | 39 |
| Average | 11 | 27 | 42 |
| Std dev | 1.47 | 2.8 | 3.58 |
| %RSD | 13.59 | 10.52 | 8.52 |
Tab. 1. Fluid bed coater processing parameters in coatings.
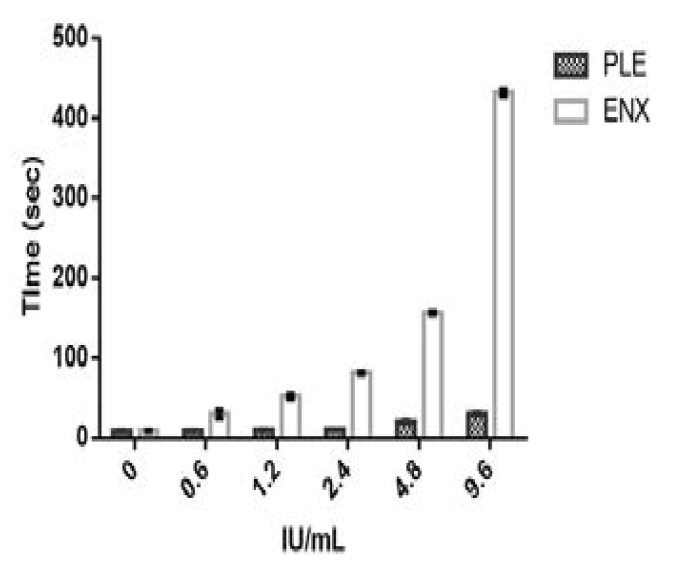
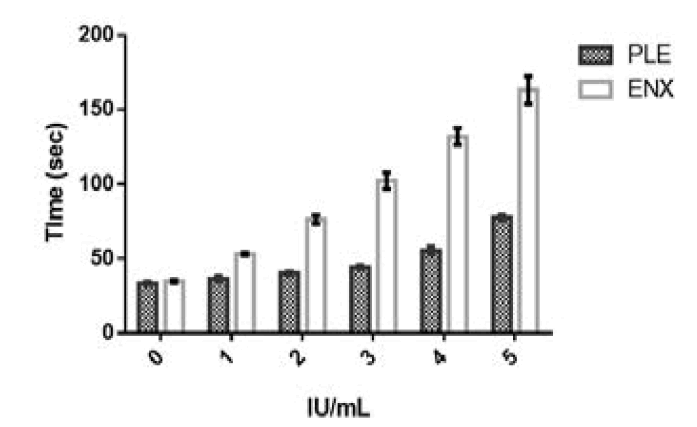
HEPTEST and aPTT tests were performed on the partially PLE fractions. The HEPTEST and aPTT activity for USP Enoxaparin sodium was more than the PLE fraction. The coagulation times for HEPTEST of PLE fraction were 3, 5, 8, 8 and 14 folds lower than USP Enoxaparin sodium for concentrations of 0.6, 1.2, 2.4, 4.8 and 9.6 IU/mL as represented in Table 2 and Figure 3. The coagulation times for aPTT of PLE fraction were 1, 2, 2, 2 and 2 folds lower than USP enoxaparin sodium for concentrations of 1, 2, 3, 4 and 5 IU/mL as represented in Table 3 and Figure 4. The PLE coagulation times for HEPTEST increased by 2 and 3 fold higher for 4.8 and 9.6 IU/mL concentration compared to control plasma. A similar pattern of 2-fold higher coagulation times was seen in 4 and 5 IU/mL concentrations for aPTT. The %RSD of HEPTEST and aPTT test for enoxaparin sodium ranged between 0.9 to 16.5 and 1.8 and 5.7 respectively. The %RSD of HEPTEST and aPTT tests for PLE ranged between 2.1 to 6.8 and 2.5 to 4.9 respectively [47-49].
| Concentration (IU/mL) | Preparation | Average | Std Dev | %RSD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| Enoxaparin sodium | 0 | 9.7 | 8.4 | 8.7 | 9.1 | 9.1 | 8.7 | 9 | 0.45 | 5.1 |
| (Plasma control) | ||||||||||
| 0.6 | 28.4 | 26.3 | 28.7 | 39.6 | 27.1 | 28.6 | 29.8 | 4.9 | 16.5 | |
| 1.2 | 56.1 | 52.6 | 55 | 50.1 | 49.5 | 51.9 | 52.5 | 2.62 | 5 | |
| 2.4 | 80.4 | 81.7 | 83.5 | 79.1 | 80.3 | 82.6 | 81.3 | 1.63 | 2 | |
| 4.8 | 157.3 | 159.5 | 154.3 | 156.5 | 154.2 | 156.7 | 156.4 | 1.99 | 1.3 | |
| 9.6 | 429.4 | 430.4 | 431.3 | 430.2 | 439.7 | 431.6 | 432.1 | 3.81 | 0.9 | |
| PLE | 0 | 8.5 | 9.7 | 8.3 | 8.1 | 8.9 | 8.7 | 8.7 | 0.57 | 6.5 |
| (Plasma control) | ||||||||||
| 0.6 | 8.3 | 8.9 | 9.5 | 9.4 | 9.2 | 8.5 | 9 | 0.49 | 5.4 | |
| 1.2 | 9.2 | 8.5 | 9.9 | 10 | 9.7 | 10.3 | 9.6 | 0.65 | 6.8 | |
| 2.4 | 10.2 | 10.3 | 10.7 | 10.2 | 10.5 | 10.6 | 10.4 | 0.21 | 2.1 | |
| 4.8 | 18.2 | 20.3 | 20.1 | 20.2 | 21.3 | 22.2 | 20.4 | 1.34 | 6.6 | |
| 9.6 | 29.7 | 28.4 | 29.4 | 30.5 | 30.4 | 31.1 | 29.9 | 0.96 | 3.2 | |
Tab. 2. Formulations of IR drug loading pellets.
Fig.3. HEPTEST Coagulation times for Enoxaparin sodium and Purified Leaf Extract (PLE). Where X-axis denotes the potency strengths in IU/ mL and Y-axis denotes the coagulation time in seconds for enoxaparin sodium and purified leaf extract. The error bars in the graph are the representation of the standard deviation.
| Concentration (IU/mL) | Preparation | Average | Std Dev | %RSD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| Enoxaparin sodium | Plasma control | 35.2 | 33.1 | 34.5 | 34.6 | 35.3 | 36.4 | 34.9 | 1.09 | 3.1 |
| 1 | 51.8 | 53.9 | 53.3 | 52 | 53.3 | 54.1 | 53.1 | 0.96 | 1.8 | |
| 2 | 71.3 | 77.1 | 78.4 | 74.5 | 78.8 | 77.5 | 76.3 | 2.86 | 3.8 | |
| 3 | 99.7 | 100.7 | 99.8 | 96 | 108.6 | 109.4 | 102.4 | 5.39 | 5.3 | |
| 4 | 135.9 | 140.3 | 128 | 127.5 | 126.5 | 133.7 | 132 | 5.54 | 4.2 | |
| 5 | 153.4 | 180.5 | 164.1 | 158 | 160.3 | 162.9 | 163.2 | 9.29 | 5.7 | |
| PLE | Plasma control | 33.8 | 32.5 | 31.6 | 35.1 | 33.9 | 33.9 | 33.5 | 1.23 | 3.7 |
| 1 | 35.7 | 33.9 | 37.3 | 38.4 | 37.9 | 35.8 | 36.5 | 1.68 | 4.6 | |
| 2 | 38.7 | 39.2 | 40.5 | 40.2 | 41.6 | 41.3 | 40.3 | 1.14 | 2.8 | |
| 3 | 42.1 | 43.8 | 43.9 | 45 | 44.8 | 46.2 | 44.3 | 1.39 | 3.1 | |
| 4 | 52.1 | 53.5 | 53.8 | 56.1 | 58.2 | 58.9 | 55.4 | 2.74 | 4.9 | |
| 5 | 79.1 | 75.3 | 75.1 | 78.3 | 78.2 | 79.5 | 77.6 | 1.91 | 2.5 | |
Tab. 3. Formulations of IR drug loading pellets.
Fig.4. aPTT Coagulation times for Enoxaparin sodium and Purified Leaf Extract (PLE). Where X-axis denotes the potency strengths in IU/ mL and Y-axis denotes the coagulation time in seconds for enoxaparin sodium and purified leaf extract. The error bars in the graph are the representation of the standard deviation.
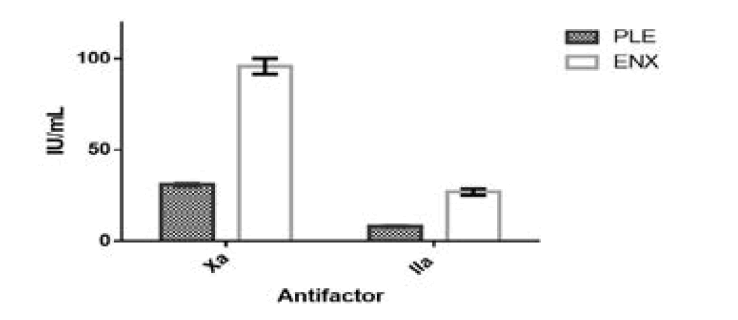
The relative potency of the partially purified leaf extracts was determined by chromogenic assay in comparison to enoxaparin sodium. The average relative potency for six preparations of enoxaparin sodium and PLE for anti-factor Xa, anti-factor IIa and the ratio of anti-factor Xa to IIa is represented in Table 4. The relative potency of Enoxaparin sodium and PLE for anti-factor Xa ranged between 92 to 101 and 30 to 32 IU/mL respectively. The relative potency of Enoxaparin sodium and PLE for anti-factor IIa ranged between 25.4 to 29.3 and 7.8 to 8.5 IU/mL respectively. The ratio of enoxaparin sodium and PLE for anti-factor Xa to antifactor IIa ranged between 3.4 to 3.8 and 3.5 to 4.1 respectively (Figure 5). The relative potency of PLE was 3 folds lower compared to enoxaparin sodium. However, the ratio of anti-factor Xa to IIa was similar.
|
Estimated potency |
Preparation |
Average |
Std Dev |
%CV |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
I |
II |
III |
IV |
V |
VI |
|
|
|
||
|
Enoxaparin Sodium |
anti-factor Xa (IU/mL) |
92 |
101 |
100 |
92 |
92 |
98 |
96 |
4.31 |
4.5 |
|
anti-factor Iia (IU/mL) |
25.4 |
29.1 |
29.3 |
25.9 |
26.9 |
25.6 |
27 |
1.76 |
6.5 |
|
|
Ratio |
3.6 |
3.5 |
3.4 |
3.6 |
3.4 |
3.8 |
3.6 |
0.15 |
4.3 |
|
|
PLE |
anti-factor Xa (IU/mL) |
30 |
30 |
31 |
32 |
32 |
31 |
31 |
0.89 |
2.7 |
|
anti-factor Iia (IU/mL) |
8.5 |
8.1 |
8.3 |
8 |
7.8 |
8.1 |
8.1 |
0.24 |
3 |
|
|
Ratio |
3.5 |
3.8 |
3.8 |
4 |
4.1 |
3.9 |
3.8 |
0.21 |
5.4 |
|
Tab. 4. Potency estimate by chromogenic assay for enoxaparin sodium and PLE.
Fig.5. Determination of relative potency in enoxaparin sodium and Purified Leaf Extract (PLE) by chromogenic assay. Where the X-axis denotes the anti-factor Xa and IIa (parameters) and the Y-axis denotes the relative potency strengths in IU/mL for enoxaparin sodium and purified leaf extract. The error bars in the graph are the representation of the standard deviation.
Conclusion
In conclusion, this study demonstrated soxhlet extraction using organic solvents and purification of the crude leaf extracts using the source Q15 fast flow resin yielded better anticoagulant properties using orthogonal approaches for an estimate of the potency. Although, the potency was not similar to Enoxaparin sodium. However, the relative potency of these extracts was prominent using orthogonal approaches.
Conflict of Interest
Authors declare no conflicts of interest.
Acknowledgment
The authors dedicate this manuscript to Late Prof P. Maruthi Mohan, department of biochemistry, Osmania university, Hyderabad, TS, India. The research work was supported by the department of science and technology (INSPIRE Faculty Award (DST) IFA12-LSPA-11to GR).
References
- Ahmed SS, Alqahtani AM, Alqahtani T, Alamri AH, Menaa F, et al. Green synthesis, characterizations of zinc oxide nanoparticles from aqueous leaf extract of Tridax procumbens Linn. and assessment of their anti-hyperglycemic activity in streptozoticin-induced diabetic rats. Materials. 2022; 15:8202.
[Crossref] [Google Scholar] [PubMed]
- Akram M, Rashid A. Anti-coagulant activity of plants: Mini review. J Thromb Thrombolysis. 2017; 44:406-411.
[Crossref] [Google Scholar] [PubMed]
- Al-Bari MA, Hossain S, Mia U, Al Mamun MA. Therapeutic and mechanistic approaches of Tridax procumbens flavonoids for the treatment of osteoporosis. Curr Drug Tar. 2020; 21:1687-1702.
[Crossref] [Google Scholar] [PubMed]
- Al Mamun MA, Asim MM, Sahin MA, Alâ?Bari MA. Flavonoids compounds from Tridax procumbens inhibit osteoclast differentiation by downâ?regulating câ?Fos activation. J Cell Mol Med. 2020; 24:2542-2551.
[Crossref] [Google Scholar] [PubMed]
- Al Mamun MA, Hosen MJ, Khatun A, Alam MM, Al-Bari MA. Tridax procumbens flavonoids: A prospective bioactive compound increased osteoblast differentiation and trabecular bone formation. Biol Res. 2017; 50:1-10.
[Crossref] [Google Scholar] [PubMed]
- Alotaibi BS, Ijaz M, Buabeid M, Kharaba ZJ, Yaseen HS, et al. Therapeutic effects and safe uses of plant-derived polyphenolic compounds in cardiovascular diseases: A review. Drug Des Devel Ther. 2021: 4713-4732.
[Crossref] [Google Scholar] [PubMed]
- Andriana Y, Xuan TD, Quy TN, Minh TN, Van TM, et al. Antihyperuricemia, antioxidant and antibacterial activities of Tridax procumbens L. Foods. 2019; 8:21.
[Crossref] [Google Scholar] [PubMed]
- Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015; 4:2167-0412.
- Ayyanar CB, Helaili S, Rangappa SM, Boonyasopon P, Siengchin S. Attempt to identify antimicrobial Tridax procumbens (TP) mechanical properties using experimental work coupled with FEM model for biomedical applications. J Mech Behav Biomed Mater. 2023; 146:106086.
[Crossref] [Google Scholar] [PubMed]
- Bhat RB, Etejere EO, Oladipo VT. Ethnobotanical studies from central Nigeria. Econ Bot. 1990; 44:382-390.
- Chen AL, Hershgold EJ, Wilson DE. One-stage assay of heparin. J Lab Clin Med. 1975; 85:843-854.
[Google Scholar] [PubMed]
- Chinnappan BA, Krishnaswamy M, Bal T, Rajora AD. In vitro-in vivo wound healing efficacy of Tridax Procumbens extract loaded Carboxymethylcellulose film. Int J Biol Macromol. 2023; 253:126695.
[Crossref] [Google Scholar] [PubMed]
- Devi K, Soni S, Tripathi V, Pandey R, Moharana B. Ethanolic extract of Tridax procumbens mitigates pulmonary inflammation via inhibition of NF-κB/p65/ERK mediated signalling in an allergic asthma model. Phytomedicine. 2022; 99:154008.
[Crossref] [Google Scholar] [PubMed]
- Diwan PV, Tilloo LD, Kulkarni DR. Influence of Tridax procumbens on wound healing. Indian J Med Res. 1982; 75:460-464.
[Google Scholar] [PubMed]
- Bhagwat DA, Killedar SG, Adnaik RS. Anti-diabetic activity of leaf extract of Tridax procumbens. Int J Green Pharm. 2008; 2.
- Fatima F, Aldawsari MF, Ahmed MM, Anwer MK, Naz M, et al. Green synthesized silver nanoparticles using Tridax procumbens for topical application: Excision wound model and histopathological studies. Pharmaceutics. 2021; 13:1754.
[Crossref] [Google Scholar] [PubMed]
- Gubbiveeranna V, Kusuma CG, Bhavana S, Sumachirayu CK, Ravikumar H, et al. Potent procoagulant and platelet aggregation inducing serine protease from Tridax procumbens extract. Pharmacogn Res. 2019; 11.
- Hossain MA, Al-Hdhrami SS, Weli AM, Al-Riyami Q, Al-Sabahi JN. Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in sultanate of Oman. Asian Pac J Trop Biomed. 2014; 4:368-372.
[Crossref] [Google Scholar] [PubMed]
- Ikese CO, Okoye ZC, Kukwa DT, Adoga SO, Lenka JL. Effect of aqueous leaf extract of Tridax procumbens on blood coagulation. Int J Pharm Sci Rev. 2015; 6:3391.
- Jachak SM, Gautam R, Selvam C, Madhan H, Srivastava A, et al. Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. Fitoterapia. 2011; 82:173-177.
[Crossref] [Google Scholar] [PubMed]
- Jin T, Chen YW, Howard A, Zhang YZ. Purification, crystallization and initial crystallographic characterization of the Ginkgo biloba 11S seed globulin ginnacin. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008; 64:641-644.
[Crossref] [Google Scholar] [PubMed]
- Jin T, Guo F, Chen YW, Howard A, Zhang YZ. Crystal structure of Ara h 3, a major allergen in peanut. Mol Immunol. 2009; 46:1796-1804.
[Crossref] [Google Scholar] [PubMed]
- Jindal A, Kumar P. Antimicrobial flavonoids from Tridax procumbens. Nat Prod Res. 2012; 26:2072-2077.
[Crossref] [Google Scholar] [PubMed]
- Joshi RK, Badakar V. Chemical composition and in vitro antimicrobial activity of the essential oil of the flowers of Tridax procumbens. Nat Prod Commun. 2012; 7:193.
[Crossref] [Google Scholar] [PubMed]
- Karthik S, Suriyaprabha R, Balu KS, Manivasakan P, Rajendran V. Influence of ball milling on the particle size and antimicrobial properties of Tridax procumbens leaf nanoparticles. IET Nanobiotechnol. 2017; 11:12-17.
[Crossref] [Google Scholar] [PubMed]
- Langdell RD, Wagner RH, Brinkhous KM. Effect of antihemophilic factor on one-stage clotting tests: a presumptive test for hemophilia and a simple one-stage antihemophilic factor assay procedure. J Lab Clin Med. 1953; 41:637-647.
[Google Scholar] [PubMed]
- Larrieu MJ, Weilland C. Use of cephalin in coagulation tests. Rev Hematol. 1957; 12:199-210.
[Google Scholar] [PubMed]
- Majekodunmi SO. Review of extraction of medicinal plants for pharmaceutical research. Res J Med Sci. 2015; 3:521-527.
- Muthukumar B, Nandini MS, Elumalai P, Balakrishnan M, Satheeshkumar A, et al. Enhancement of cell migration and wound healing by nano-herb ointment formulated with biosurfactant, silver nanoparticles and Tridax procumbens. Front Microbiol. 2023; 14:1225769.
[Crossref] [Google Scholar] [PubMed]
- Naqash SY, Nazeer RA. Anticoagulant, antiherpetic and antibacterial activities of sulphated polysaccharide from Indian medicinal plant Tridax procumbens L. (Asteraceae). Appl Biochem Biotechnol. 2011; 165:902-912.
[Crossref] [Google Scholar] [PubMed]
- Pareek H, Sharma S, Khajja BS, Jain K, Jain GC. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complement Altern Med. 2009; 9:1-7.
[Crossref] [Google Scholar] [PubMed]
- Patel C, Deoghare S. Heart failure: Novel therapeutic approaches. J Postgrad Med. 2015; 61:101-118.
[Crossref] [Google Scholar] [PubMed]
- Petchi RR, Parasuraman S, Vijaya C. Antidiabetic and antihyperlipidemic effects of an ethanolic extract of the whole plant of Tridax procumbens (Linn.) in streptozotocin-induced diabetic rats. J Base Clin Pharm. 2013; 4:88.
[Crossref] [Google Scholar] [PubMed]
- Pungle R, Nile SH, Makwana N, Singh R, Singh RP, et al. Green synthesis of silver nanoparticles using the Tridax Procumbens plant extract and screening of its antimicrobial and anticancer activities. Oxid Med Cell Longev. 2022; 2022:9671594.
[Crossref] [Google Scholar] [PubMed]
- Ravikumar V, Shivashangari KS, Devaki T. Effect of Tridax procumbens on liver antioxidant defense system during lipopolysaccharide-induced hepatitis in D-galactosamine sensitised rats. Molecular Cell Biochem. 2005; 269:131-136.
[Crossref] [Google Scholar] [PubMed]
- Rivai H, Hasni LA, Zulharmita Z. Qualitative and quantitative analysis of the content of chemical compounds from extracts of hexane, acetone, ethanol and water from avocado leaves (Persea americana Mill). World J Pharm Pharm Sci. 2019; 8:149-167.
- Salahdeen HM, Murtala BA. Vasorelaxant effects of aqueous leaf extract of Tridax procumbens on aortic smooth muscle isolated from the rat. J Smooth Muscle Res. 2012; 48:37-45.
- Salami SA, Salahdeen HM, Anidu BS, Murtala BA, Alada AA. Preliminary mechanistic study on the trachea smooth muscle relaxant activity of aqueous leaf extract of Tridax procumbens in male wistar rats. J pharmacopunct. 2022; 25:209.
[Crossref] [Google Scholar] [PubMed]
- Salami SA, Salahdeen HM, Ugbebor EC, Murtala BA, Raji Y. Effects of aqueous leaf extract of Tridax procumbens on contractile activity of corpus cavernosum in N-nitro-l-arginine methyl ester-induced hypertensive male rats. J Integr Med. 2018; 16:51-56.
[Crossref] [Google Scholar] [PubMed]
- Saxena M, Mir AH, Sharma M, Malla MY, Qureshi S, et al. Phytochemical screening and in-vitro antioxidant activity isolated bioactive compounds from Tridax procumbens Linn. Pak J Biol Sci. 2013; 16:1971-1977.
[Crossref] [Google Scholar] [PubMed]
- Singh CP, Mishra PK, Gupta SP. Design and Formulation of Tridax procumbens based Polyherbal Cream for Wound Healing Potential. Pharm Lett. 2016;8:15-21.
- Subramanian SS, Nagarajan S. Flavonoids of the seeds of Crotalaria retusa and Crotalaria striata. Cur Sci. 1969.
- Syed A, Benit N, Alyousef AA, Alqasim A, Arshad M. In-vitro antibacterial, antioxidant potentials and cytotoxic activity of the leaves of Tridax procumbens. Saudi J Biol Sci. 2020; 27:757-761.
[Crossref] [Google Scholar] [PubMed]
- Taddei A, Rosas-Romero AJ. Bioactivity studies of extracts from Tridax procumbens. Phytomedicine. 2000; 7:235-238.
[Crossref] [Google Scholar] [PubMed]
- Tiwari U, Rastogi B, Singh P, Saraf DK, Vyas SP. Immunomodulatory effects of aqueous extract of Tridax procumbens in experimental animals. J Ethnopharmacol. 2004; 92:113-119.
[Crossref] [Google Scholar] [PubMed]
- Tzima K, Brunton NP, Rai DK. Evaluation of the impact of chlorophyll removal techniques on polyphenols in rosemary and thyme byâ?products. J Food Biochem. 2020; 44:13148.
[Crossref] [Google Scholar] [PubMed]
- Yaduvanshi B, Mathur R, Mathur SR, Velpandian T. Evaluation of wound healing potential of topical formulation of leaf juice of Tridax procumbens L. in mice. Indian J Pharm Sci. 2011; 73:303.
[Crossref] [Google Scholar] [PubMed]
- Yin ET, Wessler S, Butler JV. Plasma heparin: A unique, practical, submicrogram-sensitive assay. J Lab Clin Med. 1973; 81:298-310.
[Google Scholar] [PubMed]
- L Jr YL, Darah I, Sasidharan S, Jain K. Antimicrobial activity of Emilia sonchifolia DC., Tridax procumbens L. and Vernonia cinerea L. of Asteracea family: Potential as food preservatives. Malays J Nutr. 2009; 15:223-231.
[Google Scholar] [PubMed]