Review Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 1
A comprehensive review of mechanisms, efficacy and clinical implica tions of UFH and LMWH in anticoagulant therapy: A prospective oncotarget
Ramesh Bandla1,2,4, Jaisatya Gowri Gogada1, Sugunakar Vure1, Praveen Boddana3, Abdul Qadeer Mohammed4, Rajesh Medisetty4 and Raghu Gogada1,2*2Department of Biochemistry and Plant Physiology, M.S. Swaminathan School of Agriculture (MSSSoA), Centurion University of Technology and Management (CUTM), Parlakhemundi-761211, Odisha, India
3Department of Plant Pathology, M.S. Swaminathan School of Agriculture (MSSSoA), Centurion University of Technology and Management (CUTM), Parlakhemundi, Odisha, India
4Analytical R & D Division, Biological E. Limited, Hyderabad, Telangana, India
Raghu Gogada, Department of Molecular Therapeutics, Scigenebio Pvt Ltd, Hyderabad, Telangana, India,
Received: 05-Feb-2025, Manuscript No. OAR-25-160878; , Pre QC No. OAR-25-160878; Editor assigned: 10-Feb-2025, Pre QC No. OAR-25-160878; Reviewed: 24-Feb-2025, QC No. OAR-25-160878; Revised: 01-May-2025, Manuscript No. OAR-25-160878; Published: 28-May-2025
Abstract
Administering anticoagulant is critical during the treatment and prevention of the thromboembolic disorders, with Unfractionated Heparin (UFH) and Low Molecular Weight Heparins (LMWH) being widely used agents due to their potential and anticancer properties. However, while both employ a similar mechanism acting on thrombin and factor Xa through antithrombin, differ in their pharmacokinetics and dynamics significantly. A heparin type UFH has a short half-life, needs frequent visits from the patients for the purpose of monitoring and involves a higher danger of Heparin-Induced Thrombocytopenia (HIT). In such a way LMWHs usually guarantee predictable dosage, less demand for regular tests and low risk of HIT which make them more favourable in advanced medicine practices. This article compares UFH and LMWHs in terms of effectiveness and safety, differential dosages and administration for treatment of patients with thromboembolic events such as DVT, PE and during surgical perioperative interventions. Knowing the differences of both heparins and its efficiency might be useful for taking the right decision during therapy and thereby enhancing thromboembolic therapies.
Keywords
Thromboembolic disorders; Pharmacokinetics; DVT; UFH; LMWH; HIT; PE
Introduction
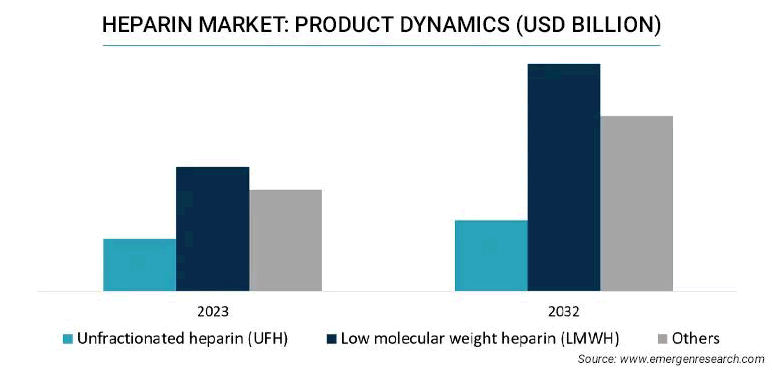
The market of global heparin was 5.58 billion USD in 2022 and is expected to register a revenue CAGR of 5.5% during the forecast period, as shown in Figure 1. More research work is going on due to its high applicability in varied therapeutics with minimum side effects, which is the main reason for driving market revenue growth. UFH and LMWH are two extensively used anticoagulants, each with its own set of pharmacological properties and therapeutic applications. UFH-a complex mixture of glycosaminoglycans- has long been the major therapy option for a variety of thromboembolic diseases. On the contrary, LMWH, which is derived from UFH through chemical or enzymatic polymerization, is becoming increasingly popular in clinical practice due to its more predictable pharmacokinetic profile and decreased risk of adverse effects. To optimize anticoagulant therapy and improve patient outcomes, a detailed understanding of the mechanisms of action, pharmacological differences and clinical indications of UFH and LMWH is required [1,2].
Fig.1. Dynamics of heparin product in billion USD.
Materials and Methods
Background on heparins
Heparins are anticoagulants that are commonly used during thromboembolic therapies. They have an anticoagulant effect by increasing the activity of antithrombin III, particularly thrombin and factor Xa. UFH and LMWH are critical components of anticoagulant therapy used during various clinical contexts, including the treatment of acute coronary syndromes, VTE, surgical and medical prophylaxis. Understanding their pharmacological properties, mechanisms of action and therapeutic indications is critical for maximising their clinical use during practice. Characteristics of both types have been presented in Table 1.
| Physical characteristics and mode of action | UFH | LMWHs |
|---|---|---|
| Molecular size | 3,000-30,000 Daltons | 4,000-6,000 Daltons |
| Source | Derived from animal mucosa (porcine intestine, bovine lung) | Derived by depolymerizing UFH to smaller fragments |
| Mode of action | Binds to AT and accelerates its inhibition of thrombin and Factor Xa equally | Binds to AT and preferentially inhibits Factor Xa over thrombin due to smaller size |
| Thrombin inhibition | Strong inhibition | Weaker inhibition |
| Factor Xa inhibition | Moderate | Strong |
| Pharmacokinetics | Short half-life (~1-2 hours), requires continuous IV infusion | Longer half-life (~4–6 hours), enables subcutaneous administration |
| Plasma protein binding | High (binds to various plasma proteins, endothelial cells, macrophages, etc.) | Low (less non-specific binding), leading to more predictable effects |
| Bioavailability | ~30% after subcutaneous administration | ~90% after subcutaneous administration |
| Onset of action | Immediate (IV) | Delayed (~4 hours) after subcutaneous administration |
| Clearance | Rapid clearance, primarily via reticuloendothelial system and kidneys | Slower clearance, mostly renal excretion |
| Half-life | 30-90 minutes (depends on dose) | 3-6 hours (depends on dose) |
Tab. 1. Physical characteristics of heparins.
Importance of anticoagulant therapy
During the management of various thromboembolic disorders, anticoagulant therapy is used to prevent the formation and propagation of blood clots which can leads to serious medical complications, including stroke, MI and VTE. UFH and LMWH are among the most utilized anticoagulants due to their wellestablished efficacy, rapid onset of action and reversibility. UFH, a heterogeneous mixture of glycosaminoglycans, has been a mainstay of anticoagulant therapy for decades. Its intravenous administration allows for immediate anticoagulation in acute settings such as myocardial infarction, DVT and pulmonary embolism. Despite the close monitoring of aPTT and the risk of HIT, UFH remains indispensable in scenarios requiring rapid onset and reversal of anticoagulation [3-5].
LMWH, derived from UFH through chemical or enzymatic depolymerization, has several advantages like predictable pharmacokinetic profile, a longer half-life and a reduced risk of adverse effects like HIT. Subcutaneous administration of LMWH allows for outpatient management of thromboembolic disorders and thromboprophylaxis in surgical and medical patients, contributing to improved patient convenience and adherence to therapy. Understanding the importance of anticoagulant therapy and the role of UFH and LMWH in preventing thromboembolic events is crucial for healthcare providers in optimizing patient outcomes and reducing the burden of thrombotic complications.
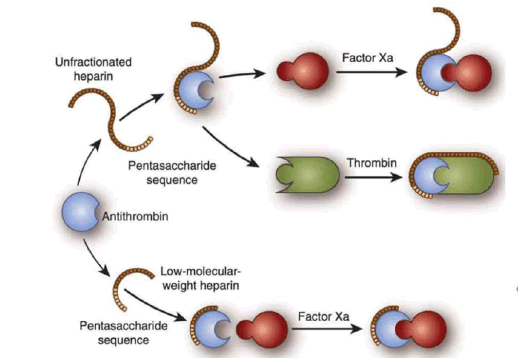
Fig.2. Synthesis of UFH and LMWH.
Overview of heparins
Heparins are a class of anticoagulants widely used as medications for the prevention and treatment of thromboembolic disorders. They are derived from porcine intestinal mucosa or bovine lung tissue made-up of sulfated glycosaminoglycans with a high negative charge. Heparins exhibit their anticoagulant effects through the enhancement of ATIII, a naturally occurring serine protease inhibitor. ATIII binds to heparin and undergoes a conformational change, leading to the accelerated inhibition of several coagulation factors, particularly IIa and Xa [8].
LMWH exhibits a more predictable pharmacokinetic profile, a longer half-life and a reduced risk of certain adverse effects compared to UFH. Both UFH and LMWH are administered parenterally and are widely used for the prevention and treatment of thromboembolic disorders, including DVT, pulmonary embolism and acute coronary syndromes. UFH is typically administered UFH is typically administered intravenously for acute anticoagulation, whereas LMWH is administered subcutaneously for prophylaxis and treatment in outpatient settings. Despite their widespread use, heparins have limitations, including the need for monitoring, the risk of bleeding and the potential for HIT. Therefore, careful dosing and monitoring are essential to optimize the efficacy and safety of heparin therapy in clinical practice [9].
Results and Discussion
Structure and composition
UFH and LMWHs comprise sulfated glycosaminoglycan chains, though they exhibit notable differences in composition and molecular characteristics. UFH consists of a heterogeneous mixture of polysaccharide chains varying in length and molecular weight, typically ranging from 15 to 20 kDa, with some chains extending up to 40 kDa. These chains are made up of repeated disaccharide units of D-glucosamine and either D-glucuronic acid or L-iduronic acid, often sulfated at various positions, contributing to UFH's heterogeneity. Conversely, LMWHs, derived from UFH through chemical or enzymatic depolymerization, feature smaller and more uniform chains with an average molecular weight between 4 and 6 kDa. While maintaining the disaccharide units present in UFH, LMWHs exhibit reduced sulfation and a more predictable size distribution of polysaccharide chains. This modification results in improved bioavailability and a more consistent pharmacokinetic profile for LMWHs compared to UFH, making them favored options in many clinical settings [10].
Pharmacokinetics and pharmacodynamics
UFH and LMWHs have different pharmacokinetic and pharmacodynamic profiles due to their differences in molecular size, composition and mode of administration. UFH is administered intravenously or subcutaneously and exhibits variable absorption, distribution, metabolism and elimination. Its large and heterogeneous polysaccharide chains interact with various plasma proteins, resulting in unpredictable pharmacokinetics. UFH primarily acts by enhancing the activity of antithrombin III, which inhibits the factors IIa and Xa. Due to its nonspecific binding, UFH requires aPTT monitoring to maintain therapeutic anticoagulation levels. UFH has a short half-life and can be rapidly reversed with protamine sulfate. LMWHs, on the other hand, have more predictable pharmacokinetics due to their smaller and more uniform molecular weight. They are administered subcutaneously and predominantly inhibit factor Xa, with minimal effects on thrombin. LMWHs carry a reduced risk of HIT and exhibit less variability in response compared to UFH. Their subcutaneous administration results in better bioavailability, a prolonged half-life and a reduced need for monitoring [11-14].
Mechanisms of anticoagulant action
UFH and LMWHs are both widely used anticoagulants with distinct mechanisms of action. Heparins exert their anticoagulant effects primarily through binding to ATIII, a natural inhibitor of coagulation proteases, particularly factor IIa and Xa. Upon binding to ATIII, heparins induce a conformational change that enhances its ability to inhibit thrombin and factor Xa. This leads to the inhibition of converting fibrinogen to fibrin, formation of thrombin and blood clot formation [15-18].
UFH exhibits rapid onset of action which requires close monitoring due to its unpredictable pharmacokinetics and variable responses among patients. LMWHs, on the other hand, have more predictable pharmacokinetics and can be administered subcutaneously either once or twice daily dosing regimen, obviating the need for frequent monitoring in most clinical settings. Additionally, LMWHs have a more selective inhibition of factor Xa compared to thrombin, which contributes to their favourable risk-benefit profile in certain patient populations, especially those with cancer-associated thrombosis.
Inhibitory effects on factors Xa and IIa
UFH and LMWHs exert their anticoagulant effects primarily through the inhibition of factors Xa and IIa. UFH and LMWHs enhances the activity of ATIII, facilitates the inhibition of factor Xa, a pivotal enzyme in the coagulation cascade responsible for converting prothrombin to thrombin. Additionally, these heparins bind to ATIII, augmenting its inhibitory effect on thrombin. Thrombin, in turn, plays a central role in converting fibrinogen to fibrin, the structural foundation of blood clots. The inhibition of factor Xa and thrombin prevents the formation of stable fibrin clots, thereby exerting anticoagulant effects. While LMWHs primarily target factor Xa, they also exhibit some inhibitory activity against thrombin, albeit to a lesser extent compared to factor Xa inhibition. This differential inhibition profile contributes to the distinct pharmacokinetic properties and clinical profiles of UFH and LMWHs [19,20].
Interaction with antithrombin III
The interaction with ATIII is fundamental to the anticoagulant mechanisms of both UFH and LMWHs. Both heparins binds to ATIII and induce conformational changes, enhancing its inhibitory activity against key coagulation factors, particularly factors IIa and Xa. Potentiality of ATIII's inhibitory effect prevents the conversion of prothrombin to thrombin and inhibits factor Xa activity and disrupts the formation of fibrin clots. UFH and LMWHs utilizes this mechanism where LMWHs exhibit a more selective inhibition of factor Xa compared to UFH. Despite these differences, the interaction with ATIII remains central to the therapeutic efficacy of both UFH and LMWHs, highlighting the importance of this mechanism in anticoagulant therapy [21].
Differences between UFH and LMWH
UFH and LMWHs are both essential anticoagulants used in clinical practice, but they differ significantly in their pharmacological characteristics and clinical applications. UFH is made up of varied polysaccharide chains with different lengths synthesizes LMWHs through depolymerization, resulting in smaller, more uniform chains. This variance leads to differences in pharmacokinetics, as UFH requires frequent monitoring due to its unpredictable absorption and clearance, whereas LMWHs has predictable pharmacokinetics and can be administered in fixed doses without routine monitoring. Additionally, UFH has a higher risk of causing HIT compared to LMWHs, making it preferable in certain patient populations. LMWHs have a longer half-life and the bioavailability is high while administered subcutaneously, allowing for less frequent dosing and simplified administration regimens. Despite these differences, both heparins exert their effects through ATIII activity enhancement, thereby leads to inhibit thrombin and factor Xa. Understanding these distinctions is crucial for selecting the most appropriate anticoagulant therapy based on individual patient characteristics and clinical indications [22-25].
Clinical applications and therapeutic monitoring
Clinical applications and therapeutic monitoring of heparins, including UFH and LMWHs, are crucial aspects of anticoagulant therapy. These are used to treat and prevent various thromboembolic disorders, with careful monitoring of their efficacy and safety during clinical practice. Here's an overview of their clinical applications and the methods used for therapeutic monitoring (Tables 2 and 3).
| Applications | UFH | LMWH |
|---|---|---|
| Off-label uses | Extracorporeal Membrane Oxygenation (ECMO), bridging therapy | DVT prophylaxis in oncology, perioperative bridging |
| Research areas | Strategies to reduce HIT risk, improved formulations with reduced variability | Extended DVT prophylaxis in cancer, reduced osteoporosis risk, new delivery mechanisms |
| Relevance in cancer | Limited, not typically used for cancer patients for long-term anticoagulation | Widely used in Cancer-Associated Thrombosis (CAT), better safety profile |
| Relevance in COVID-19 | Preferred for ICU patients due to its quick onset and reversibility | LMWHs widely used for thromboprophylaxis in non-ICU COVID-19 patients |
| Emerging therapies | Heparin derivatives with improved selectivity and reduced side effects | Newer LMWH formulations, research into oral Factor Xa inhibitors as alternatives |
Tab. 2. Clinical applications and the methods used for therapeutic monitoring.
| Field of application | UFH and LMWH |
|---|---|
| Venous Thromboembolism (VTE) Prophylaxis | DVT and PE patients who are undergoing surgery or immobilized due to medical illness |
| Anticoagulation in Acute Coronary Syndromes (ACS) | Adjunctive therapy in patients with ACS includes unstable angina and NSTEMI, to prevent recurrent ischemic events |
| Bridge therapy | Transitioned patients from oral anticoagulants (e.g., warfarin) to invasive procedures or surgery to maintain anticoagulation while minimizing bleeding risk |
| Pregnancy and antiphospholipid syndrome | LMWHs are used for anticoagulation in pregnant women with thrombophilia or a history of recurrent pregnancy, loss associated with antiphospholipid syndrome |
Tab. 3. Clinical applications and therapeutic monitoring of heparins.
Therapeutic monitoring: Under clinical assessment, in addition to laboratory monitoring, clinical assessment of bleeding and thrombotic events, as well as the patient's underlying condition and comorbidities, is essential for evaluating the safety and efficacy. Despite their widespread use and clinical efficacy, they are also associated with several challenges and limitations in clinical practice as shown in the Table 4.
| Therapeutic implications | UFH | LMWHs |
|---|---|---|
| Monitoring | Requires frequent monitoring via aPTT | Minimal monitoring (anti-Factor Xa levels in certain cases) |
| Dosing | Weight-based, variable, requires frequent adjustments | Fixed weight-based dosing, more predictable |
| Administration route | Intravenous (continuous infusion) or subcutaneous | Subcutaneous, more convenient for outpatient therapy |
| Antidote | Protamine sulfate (complete reversal) | Protamine sulfate (partial reversal, ~60%) |
| Risk of hit | Higher (up to 5%) | Lower (~0.2-1%) |
| Risk of osteoporosis | Higher with long-term use | Lower but still present with prolonged use |
| Use in pregnancy | Safe and not crosses through the placenta | Safe and preferred during pregnancy to reduce monitoring needs |
| Use in renal impairment | Preferred in severe renal impairment, as it is less renally cleared | Requires dose adjustment or avoidance in severe renal impairment |
| Cost | Generally, less expensive | Higher cost |
| Clinical indications | Acute thromboembolism, cardiac surgery, dialysis, ECMO | DVT, PE, acute coronary syndrome, cancer-associated thrombosis, outpatient anticoagulation |
Tab. 4. Challenges and limitations in clinical practice.
Efficacy, safety, challenges and limitations
Both UFH and LMWHs are effective in preventing and treating VTE, including DVT and PE. Clinical trials have shown that LMWHs have comparable or superior efficacy to UFH, with fewer bleeding complications and simplified dosing. Safety monitoring is essential to reduce adverse events such as bleeding, requiring regular assessments of renal and liver function, medication use and lab parameters like platelet count and coagulation profiles. Close communication between healthcare providers and patients, alongside patient education on bleeding signs and treatment adherence, is critical for optimizing outcomes. Although LMWHs have a lower risk of major bleeding than UFH, other adverse effects, such as alopecia, skin necrosis and hypersensitivity, may occur. Protamine sulfate partially reverses UFH effects, but no antidote exists for LMWHs, complicating the management of bleeding in emergencies. Heparins can also interfere with coagulation tests, particularly UFH, which may affect results during cardiac surgery. Finally, LMWHs require subcutaneous administration and poor adherence to treatment can impact therapeutic outcomes [26-28].
Implications for clinical practice
The use of UFH and LMWHs in anticoagulant therapy has important implications for clinical practice. LMWHs are often favored over UFH due to their predictable pharmacokinetics, lower incidence of HIT and ease of administration. When selecting therapy, clinicians must individualize dosing based on patient-specific factors, including renal function, body weight and underlying conditions. UFH typically requires aPTT monitoring, while LMWHs may require anti-factor Xa assays, particularly in patients with renal impairment or obesity. LMWHs are commonly preferred for long-term anticoagulation in conditions like VTE and pregnancy-related thromboprophylaxis due to their reduced need for monitoring and lower complication rates. Patient education is crucial, emphasizing correct dosing, recognizing signs of bleeding or thrombosis and ensuring adherence to the prescribed regimen. Instruction on selfadministration of LMWH injections is essential to improve outcomes. Studies comparing LMWHs, such as Dalteparin and Enoxaparin, with UFH demonstrate that LMWHs are as effective as UFH in preventing recurrent VTE, with a lower risk of HIT and bleeding complications. Additionally, the integration of emerging technologies, such as wearable sensors, point-of-care testing and personalized drug delivery systems, has the potential to enhance patient care and anticoagulation management. Multidisciplinary collaboration among healthcare providers is essential for optimizing treatment outcomes and ensuring patient safety [29,30].
Clinical outcomes
Clinical outcomes associated with the use of UFH and LMWHs have been extensively studied in various patient populations and clinical settings. Both heparins have proven to be highly effective in preventing VTE in high-risk populations, such as those undergoing surgery or hospitalized for medical conditions. These medications significantly reduce the incidence of symptomatic DVT, PE and VTE-related mortality.
LMWHs are often preferred as the first line of treatment for acute DVT and PE due to their predictable pharmacokinetics and lower risk of HIT compared to UFH. Their use leads to the resolution of acute thrombosis, prevention of thrombus extension and a reduced risk of recurrent VTE.
Additionally, UFH and LMWHs are commonly used as adjunctive therapies in patients with Acute Coronary Syndrome (ACS), unstable angina and NSTEMI, to prevent recurrent ischemic events. Clinical outcomes include reduced risks of death, myocardial infarction or recurrent ischemia, along with improved overall survival and cardiac function [31-35].
Influencing factors on the patients
Several factors influence the potency of UFH and LMWHs in inhibiting factor Xa and IIa. UFH consists of varied polysaccharide chains, while LMWHs have shorter, more uniform chains, giving them a higher anti-factor Xa to anti-IIa ratio. Both heparins bind to ATIII, enhancing the inhibition of factor Xa and IIa, with potency affected by molecular weight and chain length. LMWHs are administered subcutaneously with fixed dosing and predictable effects, whereas UFH requires intravenous dosing with monitoring. LMWHs are cleared by the kidneys, so renal impairment increases bleeding risk, necessitating dose adjustments. Body weight influences LMWH dosing, while UFH may require adjustments in obese patients. Additionally, medical conditions like liver disease or Disseminated Intravascular Coagulation (DIC) can alter heparin potency. Drug interactions, including those with anticoagulants, antiplatelets or medications affecting renal or hepatic function, may impact heparin efficacy [36].
Laboratory assays
Laboratory assays for assessing anti-factor Xa and IIa (thrombin) potency are crucial for monitoring the anticoagulant effects of heparins, including UFH and LMWHs.
Laboratory assays are essential for assessing the anticoagulant effects of heparins, particularly the inhibition of factor Xa and thrombin (factor IIa). The anti-factor Xa assay measures factor Xa inhibition using plasma, factor Xa and a chromogenic substrate, with reduced color intensity indicating inhibition, which is crucial for monitoring heparins. Similarly, the anti-IIa assay assesses thrombin inhibition, commonly used for UFH. Clotting assays like aPTT monitor UFH by measuring clotting time, while TT and fibrinogen assays assess clotting dynamics, such as thrombin inhibitors or fibrinogen activity. Chromogenic assays for both antifactor Xa and anti-IIa offer precise quantification by comparing substrate cleavage intensity to standard curves, with high sensitivity and specificity, making them ideal for LMWH monitoring. Standardization and calibration are vital to ensure accuracy and consistency across laboratories, using reference materials and routine instrument adjustments [37].
A challenge in developing a faster ion chromatography method with suppressed conductivity detection between LMWHs and anion exchangers, which hindered elution. Advanced analytical methods are needed to continue improving LMWH and anticoagulant monitoring techniques [38-41].
Future directions and emerging technologies
Future directions in anticoagulant therapy, including advancements in UFH and LMWHs, are focused on enhancing efficacy, safety and convenience. The development of novel anticoagulants, such as DOACs targeting specific coagulation factors like factor Xa inhibitors, direct thrombin inhibitors, provides alternatives to traditional heparin therapy with predictable pharmacokinetics and fewer interactions. Efforts are also underway to improve heparin formulations, aiming for enhanced bioavailability, longer halflives and reduced immunogenicity to reduce dosing frequency. Emerging technologies, such as point-of-care devices and wearable sensors, offer real-time coagulation monitoring for personalized therapy. Nanotechnology-based drug delivery systems target heparins to specific thrombosis sites, minimizing systemic side effects. Additionally, biosensors and biomarkers are being developed to detect thrombin generation and platelet activation, facilitating better diagnosis and monitoring. Genomic and pharmacogenomics research aims to personalize treatment based on genetic profiles, while AI and machine learning are used to predict individual responses, enabling precision medicine. Biological therapies targeting novel coagulation pathways offer potential alternatives with improved safety profiles compared to traditional heparins [42-45].
Conclusion
In conclusion, UFH and LMWHs play crucial roles in anticoagulant therapy, offering effective management of thromboembolic disorders across various clinical settings. While both UFH and LMWHs act by enhancing antithrombin III activity, their pharmacokinetic and pharmacodynamic differences impact dosing, monitoring and clinical outcomes. LMWHs, with their predictable pharmacokinetics and lower risk of adverse effects, such as HIT, are often preferred over UFH in many scenarios. However, selecting the appropriate anticoagulant therapy depends on patient-specific factors, clinical indications and other considerations. As research progresses, integrating emerging technologies and personalized approaches into practice will further enhance anticoagulant management, ensuring safety and efficacy for patients with thromboembolic disorders. Collaborative care among healthcare providers, continuous education and patient engagement remains vital to achieving optimal outcomes and improving the quality of anticoagulant therapies.
Conflicts of Interest
Authors declare no conflicts of interest.
Acknowledgment
We have not received any funding support from any extramural funding agencies to execute this work.
References
- Abballe F, Lombardi M, Maccone I, Palazzo G, Severoni A, et al. New method for low molecular weight heparin quantification in tablets by suppressed conductivity detection and cryptand column. J Pharmaceu Biomed Analy. 2008;48:467-471.
[Crossref] [Google Scholar] [PubMed]
- Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction: Results of the Thrombolysis in Myocardial Infarction (TIMI) 11B trial. Circulation. 1999;100:1593-601.
[Crossref] [Google Scholar] [PubMed]
- Atkinson TM, Hay JL, Shoushtari A, Li Y, Paucar DJ, et al. Relationship between physician-adjudicated adverse events and patient-reported health-related quality of life in a phase II clinical trial (NCT01143402) of patients with metastatic uveal melanoma. J Cancer Res Clin Oncol. 2017;143:439-445.
[Crossref] [Google Scholar] [PubMed]
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, et al. VTE, thrombophilia, antithrombotic therapy and pregnancy: antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:691-736.
[Crossref] [Google Scholar] [PubMed]
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305-1310.
[Crossref] [Google Scholar] [PubMed]
- Bertsch T, Chapelle JP, Dempfle CE, Giannitsis E, Schwab M, et al. Multicentre analytical evaluation of a new point-of-care system for the determination of cardiac and thromboembolic markers. J Clin Lab. 2010;56:37.
[Google Scholar] [PubMed]
- Blin P, Samama CM, Sautet A, Benichou J, Lignot-Maleyran S, et al. Comparative effectiveness of direct oral anticoagulants versus low-molecular weight heparins for the prevention of venous thromboembolism after total hip or knee replacement: A nationwide database cohort study. Pharmacol Res. 2019;141:201-207.
[Crossref] [Google Scholar] [PubMed]
- Buck J, Fromings Hill J, Martin A, Springate C, Ghosh B, Ashton R, Lee G, Orlowski A. Reasons for discontinuing oral anticoagulation therapy for atrial fibrillation: A systematic review. Age and ageing. 2021;50:1108-1117.
[Crossref] [Google Scholar] [PubMed]
- Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. New Eng J Med. 1997;337:447-452.
[Crossref] [Google Scholar] [PubMed]
- Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. New Eng J Med. 2019;380:1326-1335.
[Crossref] [Google Scholar] [PubMed]
- Dentali F, Marchesi C, Pierfranceschi MG, Crowther M, Garcia D, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. Thrombosis Haemostasis. 2011;106:429-438.
[Crossref] [Google Scholar] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:326-350.
[Crossref] [Google Scholar] [PubMed]
- Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: A systematic review of therapeutic trials. Obstetrics Gynecol. 2002;99:135-144.
[Crossref] [Google Scholar] [PubMed]
- Fareed J, Hoppensteadt DA, Ramacciotti E, Hull RD. Contaminants in heparins: Are all facts known? Clin Appl Thromb Hemost. 2010;16:242-243.
[Crossref] [Google Scholar] [PubMed]
- Fareed J, Jeske W, Fareed D, Clark M, Wahi R, Adiguzel C, Hoppensteadt D. Are all low molecular weight heparins equivalent in the management of venous thromboembolism? Clin Appl Thromb/Hemostasis. 2008;14:385-392.
[Crossref] [Google Scholar] [PubMed]
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:227S-2277.
[Crossref] [Google Scholar] [PubMed]
- Harenberg J, Marx S, Weiss C, Krämer R, Samama M, et al. Working party: Methods to determine rivaroxaban of the Subcommittee on Control of Anticoagulation of the ISTH. Report of the subcommittee of control of anticoagulation on the determination of the anticoagulant effects of rivaroxaban. J Thrombosis Haemostasis. 2012;10:1433-1436.
[Crossref] [Google Scholar] [PubMed]
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:188-203.
[Crossref] [Google Scholar] [PubMed]
- Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, et al. Heparin and low-molecular-weight heparin. Chest. 1998;114:489S-510.
- Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy and safety. Chest. 2001;119:64-94.
[Crossref] [Google Scholar] [PubMed]
- Kanis JA, Borgström F, Compston J, Dreinhöfer K, Nolte E, et al. SCOPE: A scorecard for osteoporosis in Europe. Arch Osteoporos. 2013;8:1-63.
[Crossref] [Google Scholar] [PubMed]
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
- Kennedy R. Bridging anticoagulation therapy with low molecular weight heparin in patients with atrial fibrillation following a stroke is associated with adverse events. Evid Based Nur. 2021;24:7.
[Crossref] [Google Scholar] [PubMed]
- Kershaw G. Performance of activated partial thromboplastin time (APTT): Determining reagent sensitivity to factor deficiencies, heparin and lupus anticoagulants. Hemostasis Thrombosis: Met Prot. 2017:75-83.
[Crossref] [Google Scholar] [PubMed]
- Kovacs MJ, Rodger M anderson DR, Morrow B, Kells G, et al. Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism: A randomized, double-blind, controlled trial. Ann Int Med. 2003;138:714-719.
[Crossref] [Google Scholar] [PubMed]
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412-421.
[Crossref] [Google Scholar] [PubMed]
- Kvasnicka J, Penka M, Kvasnicka T, Michalcova J, Kudrnova Z, et al. Guidelines of Czech Association for Thrombosis and Haemostasis of the Czech Medical Association of J. E. Purkyne for safety treatment with new oral anticoagulants (NOAC)-dabigatran etexilate, apixaban and rivaroxaban. Vnitr Lek. 2015;61:537-546.
[Google Scholar] [PubMed]
- Leo A, Winteroll S. Laboratory diagnosis of heparin-induced thrombocytopenia and monitoring of alternative anticoagulants. Clin Diagn Lab Immunol. 2003;10:731-740.
[Crossref] [Google Scholar] [PubMed]
- Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144:673-684.
[Crossref] [Google Scholar] [PubMed]
- Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab. 2006;52:217-230.
[Google Scholar] [PubMed]
- Liu DS, Newbold R, Stevens S, Wong E, Fong J, et al. Early versus postoperative chemical thromboprophylaxis is associated with increased bleeding risk following abdominal visceral resections: a multicenter cohort study. J Gastrointest Surg. 2022;26:1495-1502.
[Crossref] [Google Scholar] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-e140.
- Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, et al. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. J Biol Chem. 1992;267:12528-12538.
[Crossref] [Google Scholar] [PubMed]
- Pengo V, Denas G, Zoppellaro G, Padayattil Jose S, Hoxha A, et al. Rivaroxaban vs. warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371.
[Crossref] [Google Scholar] [PubMed]
- Petitou M, van Boeckel CA. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew Chem Int Ed Engl. 2004;43:3118-3133.
[Crossref] [Google Scholar] [PubMed]
- Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer: a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1:e37-e46.
[Crossref] [Google Scholar] [PubMed]
- Roberti R, Iannone LF, Palleria C, Curcio A, Rossi M, et al. Direct oral anticoagulants: from randomized clinical trials to real-world clinical practice. Front Pharmacol. 2021;12:684638.
[Crossref] [Google Scholar] [PubMed]
- Samama MM, Amiral J, Guinet C, Perzborn E, Depasse F. An optimised, rapid chromogenic assay, specific for measuring direct factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104:1078-1079.
[Crossref] [Google Scholar] [PubMed]
- Schlimp CJ, Solomon C, Ranucci M, Hartmann J, Schöchl H, et al. The effectiveness of different functional fibrinogen polymerization assays in eliminating platelet contribution to clot strength in thromboelastometry. Anesth Analg. 2014;118:269-276.
[Crossref] [Google Scholar] [PubMed]
- Spyropoulos AC, Raskob GE. New paradigms in venous thromboprophylaxis of medically ill patients. Thromb Haemost. 2017;117:1662-1670.
[Crossref] [Google Scholar] [Pubmed]
- Thomas O, Lybeck E, Strandberg K, Tynngard N, Schott U, et al. Monitoring low molecular weight heparins at therapeutic levels: dose-responses of and correlations and differences between aPTT, anti-factor Xa and thrombin generation assays. PLoS One. 2015;10:e0116835.
[Crossref] [Google Scholar] [PubMed]
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011;9:226-228.
[Crossref] [Google Scholar] [PubMed]
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121(4):535-555.
[Crossref] [Google Scholar] [PubMed]
- Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688-698.
[Crossref] [Google Scholar] [PubMed]
- Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92:414-417.
[Crossref] [Google Scholar] [PubMed]