Research Article - Onkologia i Radioterapia ( 2022) Volume 0, Issue 0
A comparative study between cisplatin weekly versus every 3 weeks concurrently with radiotherapy in the locally advanced head and neck squamous cell carcinomas
Walid A. Almorsy and Neseen M.Sabry*Neseen M.Sabry, Department of Clinical Oncology, Tanta University, Tanta 31527, Egypt, Email: nesreensabry1eg@yahoo.com
Received: 21-Nov-2022, Manuscript No. OAR-22-80692; , Pre QC No. OAR-22-80692(PQ); Editor assigned: 24-Nov-2022, Pre QC No. OAR-22-80692(PQ); Reviewed: 08-Dec-2022, QC No. OAR-22-80692; Revised: 15-Dec-2022, Manuscript No. OAR-22-80692; Published: 22-Dec-2022, DOI: 10.4172/1896-8961.16.S1.005
Abstract
Background and objectives: Head and neck cancers are vigorous types of cancers and they are considered among the fifth commonest cancer types globally. Among these, squamous cell carcinoma is the commonest. Although advanced treatment in head and neck cancers have been detected, insignificant and unclear improvements in patient survival were revealed. Cisplatin along with radiotherapy is used to increase the sensitivity of radiation in those patients. However, the complete patient’s benefit from cisplatin-based radio-sensitization is unclear which reflects shortage of biomarkers predicting sensitivity. Thus, this study aimed at comparing high and low dose of cisplatin treatment concomitantly administered with radiation therapy regarding safety and efficacy in patients with locally advanced head and neck squamous cell carcinoma.
Materials and methods: Forty-six patients with locally advanced HNSCC (stages 3 and 4) were included. Patients have gone thorough pre-treatment clinical evaluation such as full medical history and physical examination, CT, PET/CT and MRI. The patients have equally divided into two groups. In group A, the planned protocol was 100 mg/m2 every 3 weeks (on days 1, 22, and 43), respectively whereas in group B, cisplatin was administrated concomitantly with radiotherapy at a planned dose of 30 mg/m2 weekly.
Results: Although no significant difference was detected regarding treatment response between our studied groups, insignificant improvement in survival was detected in higher cisplatin dose in comparison with lower doses. In addition, mucositis and dysphagia were significantly higher in high-dose Cisplatin group compared to low-dose cisplatin group with no significant difference detected in other side effects.
Conclusion: High dose cisplatin concomitantly used with radiotherapy showed higher but insignificant survival in comparison with low dose cisplatin with better toxicity profile in favour of low dose protocol. We recommend future large-scale studies with multiple low dose regimens for the evaluation of benefit/hazard balance of low dose versus high dose cisplatin with concurrent radiotherapy in treating HNSCC patients.
Keywords
Cisplatin, High/ low dose cisplatin, Radiation therapy, HNSCC
Introduction
Head and neck cancers are among the most common form of cancers, globally that include many forms of tumours however, squamous cell carcinoma is the commonest. Unfortunately, there are many challenges still exist in treating head and neck cancers including surgery, radiotherapy as well as systemic therapy for the management of locally advanced disease [1]. Although both surgical and systemic advanced approaches have shown better quality of life in patients with Head and Neck Squamous Cell Carcinoma (HNSCC), only insignificant and unclear improvements in patient survival are detected. The efficacy of certain treatment regimen in this case depends on the patient’s health status, tumour site as well as its extent [1]. Several randomized controlled trials failed to assess the efficacy of targeted therapy on the survival benefits in HNSCC patients [2-4].
Cisplatin along with radiotherapy is used to increase the sensitivity of radiation since 1970 and along with combination chemotherapy in advanced disease stages. Nonetheless, the complete patient advantage from cisplatin-based radio-sensitization remains unspecified which reflects the shortage of biomarkers predicting, sensitivity to cisplatin and increase concerns about kidney toxicity and auditory loss particularly; in older patients with chronic diseases [5-7]. A dose of 100 mg/m2 of cisplatin is usually used with simultaneous radiotherapy every 3 weeks [8], yet; is associated with remarkable acute and late toxicitie. Therefore, completing these regimens is relatively poor [6]. Consequently, a lower cumulative cisplatin doses have been suggested [9-11]. Protocols have suggested weekly low dose of 40 mg/m2 cisplatin to decrease toxicities however, no sufficient evidence is detected regarding its efficacy [9].
Accordingly, the aim of this study is to assess the efficacy and safety of low dose weekly cisplatin in comparison with the standard cisplatin higher dose treatment concurrently with Intensity Modulated Radiation Therapy (IMRT) for patients with locally advanced HNSCC.
Materials and Methods
Patient’s selection
This study has concluded 46 patients with locally advanced HNSCC (stages 3 and 4). Privacy of all patients’ data was guaranteed. In this study, 97 patients were assessed for eligibility, 29 patients did not meet the criteria and 22 patients refused to participate in the study. The remaining 46 patients were randomly allocated into two groups (23 patients in each). All allocated patients were followed- up and analysed statistically.
Inclusion criteria
• Patients proved histologically as locally advanced squamous or undifferentiated carcinomas.
• Aged between 18 years to 70 years.
• Eastern Co-operative Oncology Group (ECOG) Performance Status (PS) from 0-2
• Creatinine clearance>60 ml/min.
• A written informed consent was obtained from the included patients after informing them with the benefits and risks of treatment.
Exclusion criteria
• Patients proved to have metastatic disease or previously had neo-adjuvant chemotherapy or had past history of another malignancy.
Assessment and evaluation
Pre-treatment clinical evaluation has been done to all the patients, such as full medical history and physical examination, Computed Tomography (CT), PET/CT and Magnetic Resonance Imaging (MRI) of head and neck region with intravenous contrast were done according to the physicians and clinical demands, direct flexible endoscopic examination, X-ray of the chest or thoracic CT. Other chronic diseases were evaluated and recorded. Patients were randomly assigned into two equal groups where group A (23 patients) had high dose cisplatin and group B (23 patients) had low dose cisplatin as shown in Figure 1.
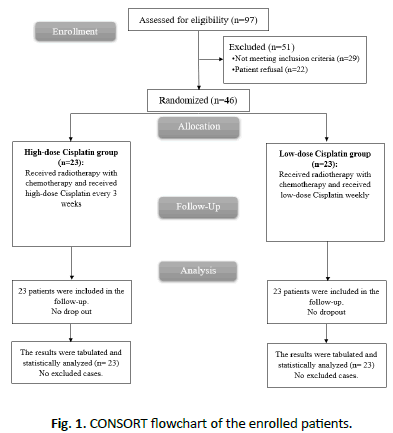
Figure 1: CONSORT flowchart of the enrolled patients.
In group A, the planned protocol was 100 mg/m2 every 3 weeks (on days 1, 22, and 43), respectively whereas in group B, cisplatin was administrated concomitantly with radiotherapy at a planned dose of 30 mg/m2 weekly.
Radiation therapy details
The patients were set up in supine position and immobilization was achieved using S-shaped head and shoulder thermoplastic mask (Aquaplast, USA). A planning CT scan with intravenous contrast was done with a slice thickness of 2.5 mm starting from the vertex of the skull down to mid-chest. The full set of images was then transferred to the eclipse treatment planning system (version 8.6). The Gross Tumour Volume (GTV) was the macroscopic disease including all positive cervical lymph nodes as detected clinically and/ or radiologically. A Clinical Target Volume (CTV’s) outlined were the high risk CTV including areas at high risk of harbouring microscopic disease and low risk CTV which included low level cervical lymph nodes in cases with node negative disease. The contouring of lymph node stations were based on many published international consensus guidelines [12]. The Planning Target Volume (PTV) margin was a 5 mm expansion from each CTV taking into consideration potential setup errors. Patients were planned for inverse IMRT with the modality of step and shoot using eclipse planning system (version 8.6, from varian medical systems). Portal image was done every week for verification of accurate treatment plan. The doses prescribed to the PTV primary (gross lesion) in high risk was 70 Gy in 2 Gy per fraction and the dose to the PTV low to intermediate risk disease was 44 Gy-50 Gy in (2 Gy per fractions) to 54 Gy-63 Gy in (1.8 Gy per fraction) 5 fractions per week.
Chemotherapy details
Regarding group A, 100 mg/m2 of cisplatin was given intravenously in 1 litre 0.9% sodium chloride for 2 hours every 3 weeks at 1st, 22nd and 43rd days. More vigorous hydration and anti-emetics were used to every 3 weeks protocol to decrease the risk of nephrotoxicity and also to decrease the emetogenic risks. For group B, cisplatin at a dose of 30 mg/m2 was administered on weekly basis in 500 ml 0.9% sodium chloride over 1 hour during the treatment course to a maximum of seven cycles. Pre\post-treatment hydration, corticosteroids, antiemetics, intravenous Mannitol 20% and supportive treatment was directed for each patient. Potassium chloride and magnesium sulfate infused over 60 minutes each was necessary with each infusion. Doses were adjusted according routine laboratory tests done before chemotherapy cycle. Chemotherapy administration was initiated if haemoglobin level was more than 10 gm/dl, platelet count of more than >100 × 109 /L, Total Leukocytic Count (TLC) more than 4.0 × 109 /L with an Absolute Neutrophilic Count (ANC) of more than 2.0 × 109 /L. Kidney function tests were evaluated frequently. Administration of at least six weekly and two out of three chemotherapy infusions in our study was defined as adequate chemotherapy exposure in groups A and B, respectively. The planned cumulative dose was 200 mg/m2.
Toxicity evaluation
Assessment of toxicity was based on the fourth version of Common Toxicity Criteria for Adverse Events [CTCAE v. 4.03] (CTCAE 2009). Scoring of acute toxicity was documented on weekly basis from the beginning of radiotherapy till 3 months post-treatment. In case of documentation of multiple occurrences, grading was based on the severest grade of that particular event. Insertion of a nasogastric tube was indicated in case of dysphagia grade 3 or more and progressive weight loss during treatment. Weekly laboratory testing was performed for all patients and chemotherapy dosing was adjusted accordingly.
Statistical analysis
Results were analysed by SPSS version 26 (IBM Inc., Chicago, IL, USA). Quantitative variables were presented as mean ± Standard Deviation (SD). Comparison between the two groups was carried out utilizing unpaired student's t-test. Qualitative variables were presented as frequency and percentage (%). Kaplan-Meier curve was used to show the survival. A two tailed P-value<0.05 was considered statistically significant.
Results
Patient characteristics (age, sex and smoking status), site and stage of tumour showed insignificant difference between both groups, as shown in Tables 1 and 2 and Figures 2 and 3.
| DWI parameters | High-dose cisplatin group (N=23) | Low-dose cisplatin group (N=23) | P value | |
|---|---|---|---|---|
| Age (years) | 59.5 ± 7.34 | 56 ± 10.21 | 0.197 | |
| Sex | Male | 13 (56.52%) | 18 (78.26%) | 0.208 |
| Female | 10 (43.48%) | 5 (21.74%) | ||
| Smoking status | 13 (56.52%) | 15 (65.22%) | 0.762 | |
| Site | Larynx | 11 (47.83%) | 5 (21.74%) | 0.26 |
| Oropharynx | 4 (17.39%) | 3 (13.04%) | ||
| Nasopharynx | 3 (13.04%) | 3 (13.04%) | ||
| Hypopharynx | 3 (13.04%) | 7 (30.43%) | ||
| Oral cavity | 2 (8.7%) | 5 (21.74%) | ||
| Stage | III | 15 (65.22%) | 12 (52.17%) | 0.434 |
| IVA | 6 (26.09%) | 10 (43.48%) | ||
| IVB | 2 (8.7%) | 1 (4.35%) | ||
Note: Data are presented as mean ± SD or frequency (%).
Tab. 1. Interobserver reproducibility in the assessment of different DWI parameters.
| Stages | High-dose cisplatin group (N=23) | Low-dose cisplatin group (N=23) | P value | |
|---|---|---|---|---|
| Tumour stage | T1 | 1 (4.35%) | 0 (0%) | 0.499 |
| T2 | 3 (13.04%) | 2 (8.7%) | ||
| T3 | 13 (56.52%) | 11 (47.83%) | ||
| T4 | 6 (26.09%) | 10 (43.48%) | ||
| Nodal stage | N0 | 3 (13.04%) | 2 (8.7%) | 0.726 |
| N1 | 18 (78.26%) | 20 (86.96%) | ||
| N2 | 2 (8.7%) | 1 (4.35%) | ||
Tab. 2. Tumour stage and nodal stage of the studied groups.
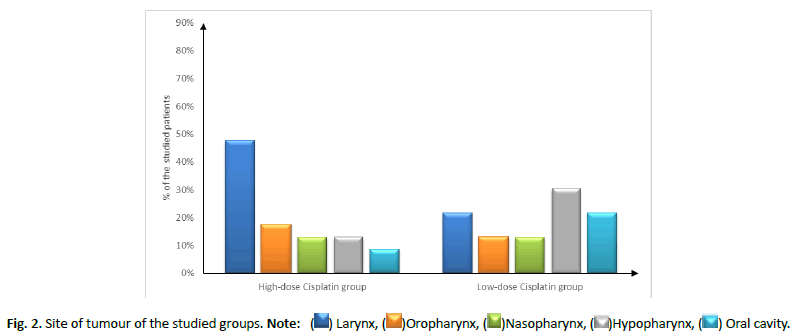
Figure 2: Site of tumour of the studied groups. Note: ( ) Larynx, (
) Larynx, ( )Oropharynx, (
)Oropharynx, ( )Nasopharynx, (
)Nasopharynx, ( )Hypopharynx, (
)Hypopharynx, ( ) Oral cavity.
) Oral cavity.
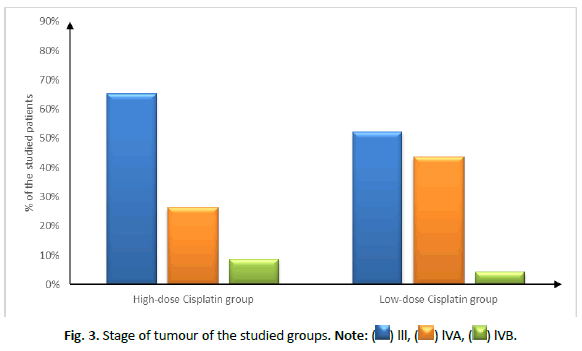
Figure 3: Stage of tumour of the studied groups. Note: ( ) lll, (
) lll, ( ) lVA, (
) lVA, ( ) lVB.
) lVB.
Regarding side effects, mucositis and dysphagia were significantly higher in high-dose cisplatin group compared to low-dose cisplatin group (P value=0.026 and 0.029; respectively), other clinical signs (nausea and vomiting, xerostomia, dermatitis, anaemia, leucopenia) were insignificantly different between both groups (Table 3) (Figure 4).
| Clinical signs | High-dose cisplatin group (N=23) | Low-dose cisplatin group (N=23) | P value | |
|---|---|---|---|---|
| Mucositis | G2 | 13 (56.52%) | 5 (21.74%) | 0.026* |
| G3 | 4 (17.39%) | 2 (8.7%) | ||
| G4 | 1 (4.35%) | 1 (4.35%) | ||
| Dysphagia | G2 | 10 (43.48%) | 5 (21.74%) | 0.029* |
| G3 | 6 (26.09%) | 2 (8.7%) | ||
| G4 | 2 (8.7%) | 1 (4.35%) | ||
| Nausea and vomiting | G2 | 8 (34.78%) | 7 (30.43%) | 0.682 |
| G3 | 5 (21.74%) | 5 (21.74%) | ||
| G4 | 3 (13.04%) | 1 (4.35%) | ||
| Xerostomia | G2 | 5 (21.74%) | 2 (8.7%) | 0.633 |
| G3 | 6 (26.09%) | 6 (26.09%) | ||
| Dermatitis | G2 | 10 (43.48%) | 12 (52.17%) | 0.593 |
| G3 | 3 (13.04%) | 1 (4.35%) | ||
| Anaemia | G2 | 6 (26.09%) | 9 (39.13%) | 0.067 |
| G3 | 10 (43.48%) | 3 (13.04%) | ||
| Leukopenia | G2 | 10 (43.48%) | 6 (26.09%) | 0.226 |
| G3 | 3 (13.04%) | 6 (26.09%) | ||
| Thrombocytopenia | G2 | 5 (21.74%) | 3 (13.04%) | 1 |
| G3 | 1 (4.35%) | 0 (0%) | ||
Note: Data are presented as frequency (%). *: Statistically significant as P value <0.05.
Tab. 3. Clinical signs of the studied groups.
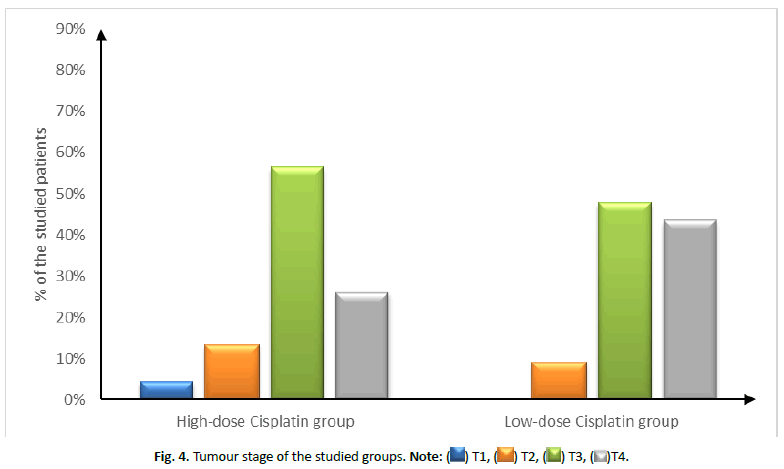
Figure 4: Tumour stage of the studied groups. Note: ( ) T1, (
) T1, ( ) T2, (
) T2, ( ) T3, (
) T3, ( )T4.
)T4.
The mean (± SE) survival was 33.13 ± 1.22 months in high-dose cisplatin group and 31.79 ± 1.96 months in low-dose cisplatin group showing insignificant difference (P value= 0.649). The Hazard Ratio (HR (95% CI)) of mortality was 1.32 (0.39-4.41) in lowdose cisplatin group compared to high-dose cisplatin group (Figures 5 and 6). While the cumulative mortality (%) at three years was 21.74% in high-dose cisplatin group compared to 26.09% in low-dose cisplatin group and was insignificantly different between both groups (P value+0.729) (Tables 4 and 5 and Figure 7).
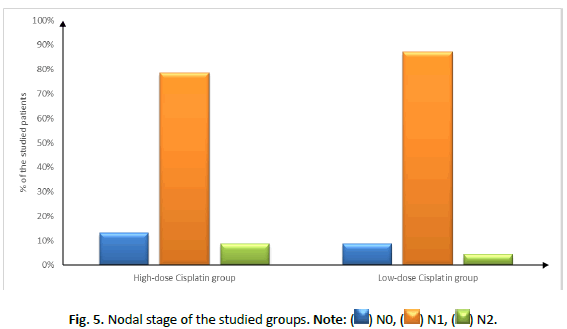
Figure 5: Nodal stage of the studied groups. Note: ( ) N0, (
) N0, ( ) N1, (
) N1, ( ) N2.
) N2.
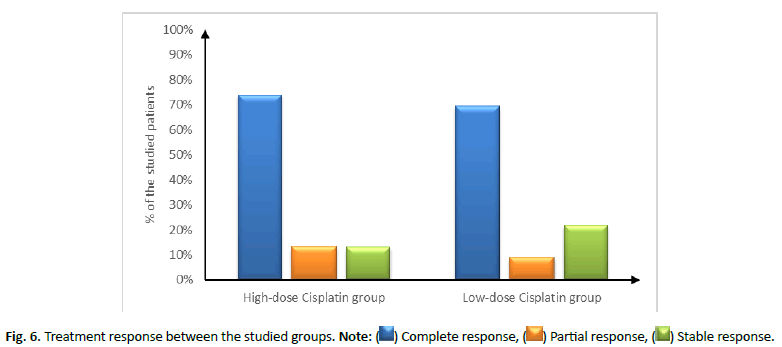
Figure 6: Treatment response between the studied groups. Note: ( ) Complete response, (
) Complete response, ( ) Partial response, (
) Partial response, ( ) Stable response.
) Stable response.
| Treatment response | High-dose cisplatin group (N=23) | Low-dose cisplatin group (N=23) | P value |
|---|---|---|---|
| Complete response | 17 (73.91%) | 16 (69.57%) | 0.694 |
| Partial response | 3 (13.04%) | 2 (8.7%) | |
| Stable disease | 3 (13.04%) | 5 (21.74%) |
Note: Data are presented as frequency (%).
Tab. 4. Treatment response between the studied groups.
| Standard deviation | High-dose cisplatin group (N=23) | Low-dose cisplatin group (N=23) | P value |
|---|---|---|---|
| Mean (95% CI) | 33.13 (30.75-35.51) | 31.79 (27.96-35.63) | 0.649 |
| SE | 1.22 | 1.96 |
Note: CI: Confidence Interval, SE: Standard Error, HR: Hazard Ratio
Tab. 5. Three-years overall survival of the studied group regarding the dose of cisplatin.
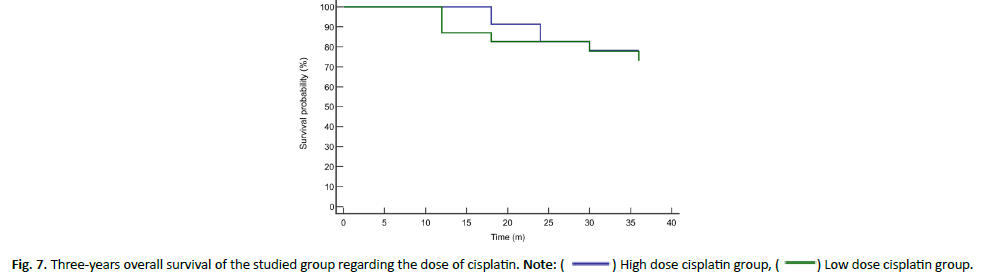
Figure 7: Three-years overall survival of the studied group regarding the dose of cisplatin. Note: ( ) High dose cisplatin group, (
) High dose cisplatin group, ( ) Low dose cisplatin group.
) Low dose cisplatin group.
Discussion
HNSCC is the fifth most common cancer worldwide [8], the disease requires vigorous management approaches including a combination of surgery, radiotherapy and chemotherapy. Nevertheless, about 50% of HNSCC patients develop locoregional treatment failure [2,13]. In addition, EORTC 22931 trials stated that 5-year overall survival was 53% in chemo-radiotherapy in comparison to 40% using radiotherapy alone [14] which remains low suggesting the discovery of novel treatment options with higher overall survival rates and lower side effects for patients with HNSCC.
In this study, comparison between low dose weekly cisplatin (30 mg/m2) and higher doses (100 mg/m2) every 3 weeks revealed insignificant difference in treatment response between both groups. However, high dose cisplatin showed higher rate of complete response (73.91%) in comparison to (69.57%) in low dose cisplatin with similar attitude between both groups in partial response. Nevertheless, low dose cisplatin showed higher insignificant stable disease status (21.74%) in comparison to higher doses of cisplatin (13.04%). In the contrary, Cooper et al., study revealed that rate of stable disease was significantly higher in cisplatin-radiotherapy combined-therapy (100 mg/m2) with an estimated two-year rate of local and regional control of 82% in comparison with 72% radiotherapy alone [15]. In the same line, higher dose of cisplatin (150 mg/m2) proved efficacy in T4 squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx or larynx along with concurrent radiation therapy (70 Gy, 2.0 Gy/fraction, daily for 5 days over 7 weeks). The study revealed a complete response rate in 85% at the 1 ry site and 88% at nodal regions [16]. The controversial results could be due to the comparison between high dose cisplatin with concurrent radiotherapy and radiotherapy alone not with low dose cisplatin with simultaneous radiotherapy.
Nonetheless, many studies have revealed the higher toxicity associated with high dose cisplatin [15,17,18]. This matches our study findings where higher incidence of mucositis and dysphagia were significantly detected in high-dose cisplatin group compared to low-dose cisplatin group. Similarly, one study has assessed patients with HNSCC between 1992 and 1999. The study concluded 295 patients and was early ceased as grade 3 or worse toxicity was detected in 89% of patients who were on high dose cisplatin and radiotherapy in comparison to radiotherapy alone. However, the study has concluded that addition of simultaneous high-dose cisplatin to conservative single daily fractionated radiation significantly progresses survival [19]. Moreover, Bernier et al., detected higher incidence of severe (grade 3 or higher) adverse events in functional mucosal, muscular fibrosis, and cytopenia as well as nausea/vomiting in cisplatin-radiotherapy group [17]. Similar results were stated by Cooper et al., study where combined chemotherapy and radiotherapy augmented the occurrence of severe adverse events from 34% to 77% (P<0.001) in comparison with radiation therapy alone [20].
Furthermore, overall survival rates were calculated in this study and revealed insignificant difference between both groups nevertheless, longer overall survival was detected in high dose cisplatin (33.13 ± 1.22 months) in comparison to (31.79 ± 1.96 months) in low-dose cisplatin group. The Hazard Ratio of mortality increased with high-dose cisplatin group in comparison to low-dose cisplatin group. Nonetheless, the cumulative mortality (%) at three years was 21.74% in high-dose cisplatin group compared to 26.09% in low-dose cisplatin group and was insignificantly different between both groups (P value=0.729). In the contrary, one meta-analysis has compared 52 studies including 4,209 patients regarding chemoradiation regimens with weekly low-dose and three-weekly high-dose cisplatin regimens. The meta-analysis concluded no significant difference in treatment efficacy presented as overall survival or response rate between both groups. However, the weekly low dose cisplatin regimen showed more convenient and significantly less toxic with respect to severe (grade 3-4) myelosuppression (leukopenia p=0.0083; neutropenia p=0.0024), severe nausea and/or vomiting (p<0.0001), as well as severe nephrotoxicity (p=0.0099). Moreover, the weekly regimen induced more grade 3-4 dysphagia (p=0.0026) and weight loss (p<0.0001). The study could not conclude similar efficacy of both cisplatin doses [21]. Unfortunately, our study could not reveal any significant difference among both groups regarding these side effects except for mucositis and dysphagia. Furthermore, 2901 patients were included in another study where 2200 received high dose cisplatin (mean initial dose 100 mg/ m2) while the remaining had low dose cisplatin of 40 mg/m2. The study revealed similar improvement in overall survival between low and high dose cisplatin (hazard ratio=0.94, 95% confidence interval=0.80 to 1.04). However, high cisplatin dose was associated with an upsurge in acute kidney injury, neutropenia, dehydration/ electrolyte disturbance, and hearing loss [22]. Similar results were revealed in Kiyota et al., study where 261 locally advanced HNSCC patients were enrolled (3-weekly cisplatin, 132 patients; weekly cisplatin, 129 patients).The study revealed non-inferiority of weekly chemoradiotherapy to 3-weekly cisplatin in regards to overall survival, with a hazard ratio of 0.6 (99.1% CI, 0.374 to 1.273 [<1.32]) [23]. Additionally, Grade 3 or more neutropenia and infection associated with higher doses of cisplatin (49%) were detected less frequently in low dose weekly regimen (35%) as were kidney dysfunction and auditory impairment. Surprisingly, no treatment-related mortality was detected in the 3-weekly cisplatin regimen in comparison with two cases (1.6%) in the weekly cisplatin regimen which agrees with the study regarding cumulative mortality risk assessment following 3-weekly dose of cisplatin. This could owe to a limitation of the Kiyota et al., study where a noninferiority margin was pre-determined to be 10% for 5-year overall survival, equal to a noninferiority margin of HR of 1.32 which allows for 32% death upsurge in chemoradiotherapy with weekly cisplatin. This could limit the detection of higher mortality rates in advanced disease status including poorly differentiated subtypes and large tumors (pT3-4).
Conclusion
High dose cisplatin concomitantly used with radiotherapy showed higher but insignificant survival in comparison with low dose cisplatin with better toxicity profile in favour of low dose protocol. Future large-scale studies are recommended with multiple low dose regimens for further assessment of benefit/hazard ratio of low dose versus high dose cisplatin with concurrent radiotherapy in treating HNSCC patients. Although there was no discernible difference in treatment response between the groups we evaluated, there was a negligible increase in survival when larger dosages of cisplatin were used in comparison to lower doses. There was no discernible difference in the side effects between the high-dose and low-dose Cisplatin groups, except that mucositis and dysphagia were much more common in the former.
References
- Routila J, Qiao X, Weltner J, et al. Cisplatin overcomes radiotherapy resistance in OCT4-expressing head and neck squamous cell carcinoma. Oral Oncol. 2022;127:105772.
[Crossref] [Google scholar] [Pubmed]
- Argiris A, Li S, Savvides P, et al. Phase III randomized trial of chemotherapy with or without bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. 2019;37:3266-3274.
[Crossref] [Google scholar] [Pubmed]
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet. 2019;394:1915-1928.
[Crossref] [Google scholar] [Pubmed]
- Forster MD, Dillon MT, Kocsis J, et al. Patritumab or placebo, with cetuximab plus platinum therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: A randomised phase II study. Eur J Cancer. 2019;123:36-47.
[Crossref] [Google scholar] [Pubmed]
- Blanchard P, Baujat B, Holostenco V, et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol. 2011;100:33-40.
[Crossref] [Google scholar] [Pubmed]
- Forastiere AA. Is there a new role for induction chemotherapy in the treatment of head and neck cancer? J Natl Cancer Inst. 2004;96:1647-1649.
[Crossref] [Google scholar] [Pubmed]
- Pignon JP, Maître A le, Maillard E, et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother and Oncol. 2009;92:4–14.
[Crossref] [Google scholar] [Pubmed]
- Osman N, Elamin YY, Rafee S, et al. Weekly cisplatin concurrently with radiotherapy in head and neck squamous cell cancer: a retrospective analysis of a tertiary institute experience. Eur Arch Otorhinolaryngol. 2014;271:2253–2259.
[Crossref] [Google scholar] [Pubmed]
- Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–4673.
[Crossref] [Google scholar] [Pubmed]
- Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol. 2000;18:1458-1464.
[Crossref] [Google scholar] [Pubmed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with american joint committee on cancer/international union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730-6738.
[Crossref] [Google scholar] [Pubmed]
- Hall WA, Paulson E, Davis BJ, et al. NRG oncology updated international consensus atlas on pelvic lymph node volumes for intact and postoperative prostate cancer. Int J Radiat Oncol Biol Phy. 2021;109:174-185.
[Crossref] [Google scholar] [Pubmed]
- Szturz P, Cristina V, Herrera Gómez RG, et al. Cisplatin eligibility issues and alternative regimens in locoregionally advanced head and neck cancer: recommendations for clinical practice. Front Oncol. 2019;9:464.
[Crossref] [Google scholar] [Pubmed]
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843-850.
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
[Crossref] [Google scholar] [Pubmed]
- Robbins KT, Kumar P, Harris J, et al. Supradose intra-arterial cisplatin and concurrent radiation therapy for the treatment of stage IV head and neck squamous cell carcinoma is feasible and efficacious in a multi-institutional setting: results of radiation therapy oncology group trial 9615. J Clin Oncol. 2005;23:1447-1454.
[Crossref] [Google scholar] [Pubmed]
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
[Crossref] [Google scholar] [Pubmed]
- Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582-3589.
[Crossref] [Google scholar] [Pubmed]
- Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92-98.
[Crossref] [Google scholar] [Pubmed]
- Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/Intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phy. 2012;84:1198-1205.
[Crossref] [Google scholar] [Pubmed]
- Szturz P, Wouters K, Kiyota N, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: A systematic review and meta-analysis of aggregate data. The Oncologist. 2017;22:1056-1066.
[Crossref] [Google scholar] [Pubmed]
- Bauml JM, Vinnakota R, Anna Park YH, et al. Cisplatin every 3 weeks versus weekly with definitive concurrent radiotherapy for squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 2019;111:490-497.
[Crossref] [Google scholar] [Pubmed]
- Kiyota N, Tahara M, Mizusawa J, et al. Weekly cisplatin plus radiation for postoperative head and neck cancer (JCOG1008): A multicenter, noninferiority, phase II/III randomized controlled trial. J Clin Oncol. 2022;40:1980-1990.
[Crossref] [Google scholar] [Pubmed]



