Research Article - Onkologia i Radioterapia ( 2025) Volume 19, Issue 6
A Comparative Analysis for DQA of VMAT Treatment Plans using ArcCHECKTM Diode Array and EPID Portal Dosimetry
Sang-Wook Lee1, Yi-Seong Lee2 and Jeong-Koo Kim3*2Department of Radiation Oncology, Catholic Kwandong University International St. Mary's Hospital, Incheon, South Korea
3Department of Radiological Science, Hanseo University, Chungcheongnam-Do, South Korea
Jeong-Koo Kim, Department of Radiological Science, Hanseo University, Chungcheongnam-Do, South Korea, Email: jkkim@hanseo.ac.kr
Received: 05-Jul-2025, Manuscript No. OAR-25-167637; , Pre QC No. OAR-25-167637 (PQ); Editor assigned: 09-Jul-2025, Pre QC No. OAR-25-167637 (PQ); Reviewed: 21-Jul-2025, QC No. OAR-25-167637; Revised: 25-Jul-2025, Manuscript No. OAR-25-167637 (R); Published: 30-Aug-2025
Abstract
Background: As Volumetric Modulated Arc Therapy (VMAT) treatment plans become more complex and highly modulated, robust and precise dosimetric quality assurance (DQA) is essential to ensure accurate and safe radiation dose delivery. Objective: This study aimed to evaluate the effectiveness of DQA for VMAT plans delivered with the Halcyon™ linear accelerator, by comparing the performance of the ArcCHECK™ diode array and EPID-based portal dosimetry. Methods: DQA was conducted for six patients with tumors at various anatomical sites. Gamma passing rates were evaluated using both 3%/3 mm and 2%/2 mm gamma criteria, with ArcCHECK™ and EPID portal dosimetry on the Halcyon™ system. Results: The ArcCHECK™ system yielded average gamma passing rates of 99.50 ± 0.414% (3%/3 mm) and 98.50 ± 0.485% (2%/2 mm). EPID portal dosimetry produced comparable results, with passing rates of 99.01 ± 0.260% and 98.30 ± 0.165%, respectively. Statistical comparisons revealed significant differences in gamma passing rates between ArcCHECK™ and EPID in some tumor sites, but both systems showed high dosimetric agreement (mean >98%), making them suitable for clinical DQA of VMAT plans. Conclusion: Both ArcCHECK™ and EPID portal dosimetry demonstrated high accuracy and consistency in DQA for VMAT treatment planning with the Halcyon™ linear accelerator. These results confirm their clinical applicability as complementary and reliable tools in radiation therapy QA.
Keywords
Delivery quality assurance, Volumetric modulated arc therapy, ArcCHECK™ diode array, EPID portal dosimetry, Gamma passing rate
INTRODUCTION
According to the 2017 National Cancer Registry statistics from the Korea Central Cancer Registry, the five-year cancer survival rate reached 70.4%, with a 16.7% improvement in the ten-year survival rate [1]. These improvements are largely attributed to advances in surgery, chemotherapy, and particularly radiation therapy.
Technological developments in radiation therapy have enabled more precise dose delivery by reducing target margins and minimizing exposure to surrounding normal tissues and organs [2]. Image-Guided Radiation Therapy (IGRT) enhances setup accuracy by correcting positional errors based on pre-treatment imaging, thereby reducing the setup margin (SM) [3]. Similarly, Respiratory-Gated Radiation Therapy (RGRT) allows radiation to be delivered at specific phases of the respiratory cycle, effectively reducing the internal margin (IM).
Further innovations, such as Intensity-Modulated Radiation Therapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT), utilize Multi-Leaf Collimators (MLC) to create highly conformal dose distributions tailored to tumor shape and location [4,5]. Among the latest systems, the Halcyon™ linear accelerator is optimized for high-speed, image-guided VMAT delivery. It delivers radiation up to four times faster than conventional linear accelerators, improving treatment accuracy while reducing session duration and intrafraction motion [6–8].
The Halcyon™ system supports various cancer types including breast, prostate, head and neck, lung, hepatopancreatic, and rectal and offers clinical outcomes comparable to surgical resection with potentially fewer side effects. This accelerator employs a high dose rate (800 MU/s) Flattening Filter-Free (FFF) beam, reducing out-of-field dose and simplifying the beam path. However, the FFF mode may increase surface dose due to electron contamination and beam hardening effects [9–11].
As VMAT plans become more complex and highly modulated, robust and accurate dosimetric quality assurance (DQA) is essential to verify the consistency between calculated and delivered dose distributions. The gamma index method is widely used for this purpose, providing a quantitative comparison and detecting dose mismatches or geometric errors through variations in gamma passing rates [12].
Recently, Electronic Portal Imaging Device (EPID)-based portal dosimetry has been widely adopted for patient-specific QA on the Halcyon™ platform. EPID dosimetry offers high-resolution, real-time, two-dimensional dose verification and is particularly sensitive to spatial and geometric deviations [13–15]. However, its limitation lies in the inability to perform full three-dimensional dose evaluations, which are often required in rotational therapies like VMAT.
To address this limitation, various 3D dosimetry tools have been developed. Among them, the ArcCHECK™ diode array featuring a cylindrical configuration-enables multi-angle beam measurement and is widely used in rotational dose verification for VMAT, RapidArc, and tomotherapy plans [16].
In this study, we evaluated the effectiveness of ArcCHECK™ and EPID portal dosimetry in DQA for VMAT plans delivered with the Halcyon™ linear accelerator, aiming to validate the accuracy and clinical applicability of both systems.
MATERIALS AND METHODS
Materials
This study was conducted as an experimental analysis. The Halcyon™ linear accelerator is equipped with an MLC specifically optimized for IMRT and VMAT, characterized by low radiation leakage and transmission. The MLC operates at a leaf speed of up to 5 cm/s, enabling faster dose delivery compared to conventional linear accelerators and minimizing intra-fraction motion errors during treatment. The dual-layer MLC structure enables precise dose modulation, allowing therapeutic radiation to be concentrated at the tumor site while minimizing exposure to surrounding healthy tissues and thereby reducing potential side effects [13]. Additionally, the Halcyon™ system incorporates advanced image-guidance algorithms that allow real-time patient positioning adjustments, which are critical for treating complex tumor geometries, enhancing treatment accuracy, and improving patient comfort. Its simplified design compared to traditional linear accelerators also contributes to easier machine maintenance and more efficient workflow in the clinical environment [10] (Figure 1). The amorphous silicon electronic portal imaging device (a-Si EPID) used in this study exhibits less than 0.5% variation in response over a two-year period for [4–6] MV photon beams. The detector's response is proportional to the integrated dose and independent of the dose rate. However, the EPID may show hypersensitivity to low-energy photons due to scattering within the imager’s bulk layer [14,17]. Consequently, EPID response may be affected by beam softening at off-axis positions and beam hardening effects caused by patient or phantom thickness [18].

Figure 1: External view of the HalcyonTM linear accelerator system.
The ArcCHECK™ diode array (Sun Nuclear, Melbourne, FL) is a cylindrical three-dimensional dosimetry device commonly used for pre-treatment DQA in IMRT, VMAT, stereotactic radiosurgery (SRS), and other rotational therapies. It has a diameter and length of 21 cm and contains 1,386 diodes arranged helically at 1 cm intervals. Each diode has a sensitive volume of 0.0019 mm³ and is embedded 2.9 cm beneath the cylinder surface. The system samples measurements at a post-acquisition frequency of 50 ms. The accompanying SNC Patient™ software (Ver. 6.1.1.x, Sun Nuclear, Melbourne, FL) compares the measured dose data with the planned dose distribution for quantitative analysis [16] (Figure 2).
Figure 2: External view of the ArcCHECKTM diode array phantom.
Methods
Treatment planning was performed for six patients with tumors located at different anatomical sites, using the Halcyon™ linear accelerator (Varian Medical Systems, Palo Alto, CA; Version 3.0). All treatment plans were generated with the Eclipse Treatment Planning System (Version 15.6, Varian Medical Systems).
Patient-specific DQA was carried out using both the ArcCHECK™ diode array and EPID portal dosimetry on the Halcyon™ system. The calculated dose distributions from the treatment plans were exported and mapped for comparison with the measured data. Dose validation was assessed using gamma analysis with 3%/3 mm and 2%/2 mm gamma criteria. Gamma passing rates were evaluated for each case, and variations were analyzed according to tumor site and size (Figures 3, 4).
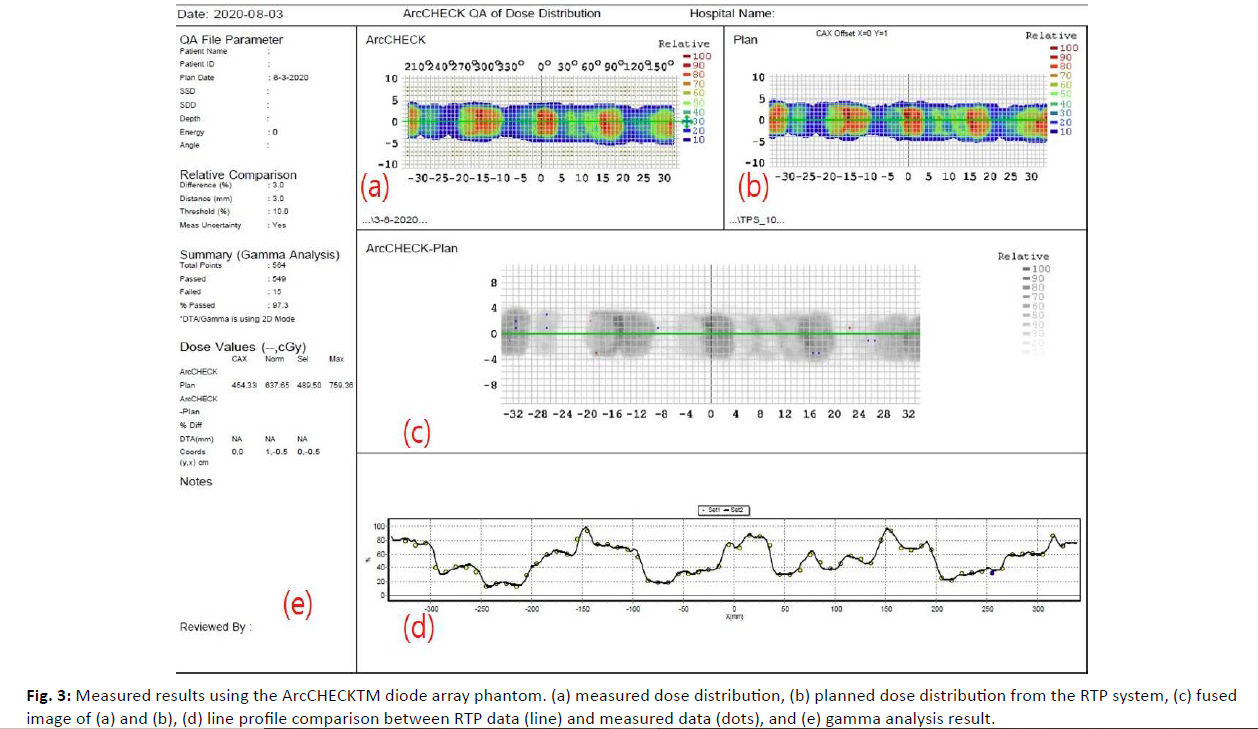
Figure 3: Measured results using the ArcCHECKTM diode array phantom. (a) measured dose distribution, (b) planned dose distribution from the RTP system, (c) fused image of (a) and (b), (d) line profile comparison between RTP data (line) and measured data (dots), and (e) gamma analysis result.
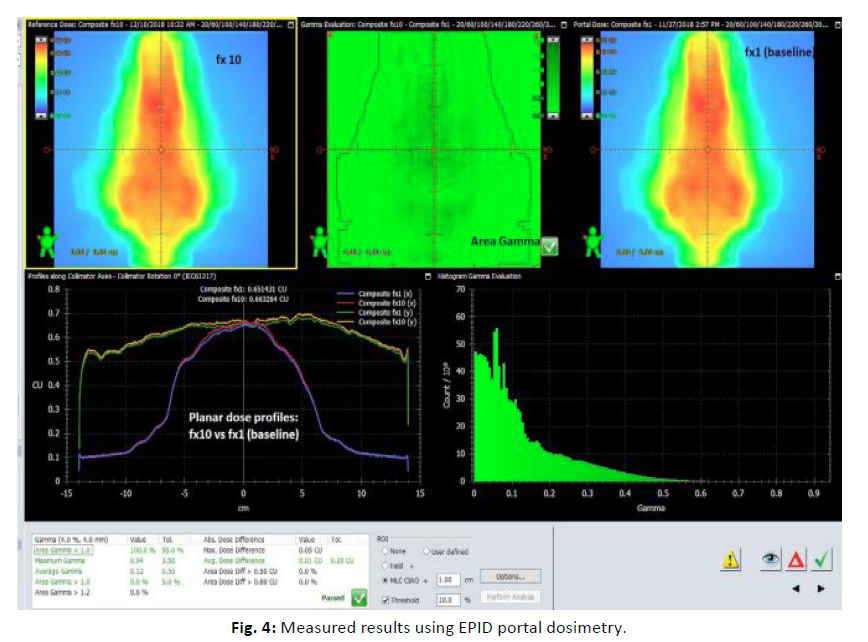
Figure 4: Measured results using EPID portal dosimetry.
RESULTS
DQA was conducted for VMAT treatment plans delivered using the Halcyon™ linear accelerator. The ArcCHECK™ diode array and EPID portal dosimetry system were employed to evaluate the gamma passing rates using two sets of criteria: 3%/3 mm and 2%/2 mm.
The ArcCHECK™ diode array recorded an average gamma passing rate of 99.50 ± 0.414% using the 3%/3 mm gamma criteria and 98.50 ± 0.485% using the 2%/2 mm gamma criteria. Among the different tumor sites, lung cancer showed the highest average gamma passing rates for both criteria: 100.00 ± 0.008% (3%/3 mm) and 99.18 ± 0.183% (2%/2 mm). In contrast, breast cancer exhibited the lowest average gamma passing rates: 98.72 ± 0.264% (3%/3 mm) and 97.62 ± 0.212% (2%/2 mm) (Table 1, 2).
| Tumor Site | ArcCHECKTM diode array | |||||
|---|---|---|---|---|---|---|
| Gamma passing rates (3%/3mm) | Average±SD | |||||
| Neck | 99.81 | 99.80 | 99.80 | 99.80 | 99.76 | 99.79±0.019 |
| Lung | 100.00 | 100.00 | 99.98 | 100.00 | 100.00 | 100.00±0.008 |
| Breast | 99.20 | 98.50 | 98.60 | 98.80 | 98.50 | 98.72±0.264 |
| Esophageal | 99.81 | 99.81 | 99.61 | 99.75 | 99.52 | 99.70±0.116 |
| Prostate | 99.31 | 99.01 | 99.31 | 99.52 | 99.31 | 99.29±0.163 |
| Rectal | 99.35 | 99.61 | 99.62 | 99.59 | 99.42 | 99.52±0.111 |
| Mean | 99.50±0.414 | |||||
Table 1: Gamma passing rates of ArcCHECKTM diode array using 3%/3mm gamma criteria
| Tumor Site | ArcCHECKTM diode array | |||||
|---|---|---|---|---|---|---|
| Gamma passing rates (2%/2mm) | Average±SD | |||||
| Neck | 98.70 | 98.90 | 98.10 | 98.30 | 98.70 | 98.54±0.294 |
| Lung | 99.40 | 99.40 | 99.00 | 99.00 | 99.10 | 99.18±0.183 |
| Breast | 97.79 | 97.90 | 97.60 | 97.50 | 97.30 | 97.62±0.212 |
| Esophageal | 98.87 | 98.91 | 98.87 | 98.63 | 98.87 | 98.83±0.101 |
| Prostate | 98.76 | 98.02 | 98.61 | 98.98 | 98.59 | 98.59±0.318 |
| Rectal | 98.71 | 98.21 | 97.90 | 98.35 | 98.12 | 98.26±0.269 |
| Mean | 98.50±0.485 | |||||
Table 2: Gamma passing rates of ArcCHECKTM diode array using 2%/2mm gamma criteria
The ArcCHECK™ diode array demonstrated strong advantages in accurately assessing rotational radiation therapy. Meanwhile, the EPID portal dosimetry yielded comparable results to ArcCHECK™, with an average gamma passing rate of 99.01 ± 0.260% using 3%/3 mm gamma criteria and 98.30 ± 0.165% using 2%/2 mm gamma criteria. Notably, for breast cancer cases, EPID portal dosimetry showed a higher gamma passing rate than the ArcCHECK™ diode array in both gamma criteria settings (Table 3, 4).
| Tumor Site | EPID portal dosimetry | |||||
|---|---|---|---|---|---|---|
| Gamma passing rates (3%/3mm) | Average±SD | |||||
| Neck | 99.01 | 99.01 | 99.02 | 99.22 | 99.22 | 99.10±0.101 |
| Lung | 98.81 | 98.82 | 98.81 | 98.86 | 98.83 | 98.83±0.019 |
| Breast | 98.88 | 98.82 | 98.96 | 98.92 | 98.93 | 98.90±0.049 |
| Esophageal | 99.45 | 99.52 | 99.42 | 99.58 | 99.76 | 99.55±0.121 |
| Prostate | 98.90 | 98.90 | 98.90 | 98.80 | 98.90 | 98.88±0.040 |
| Rectal | 98.28 | 98.93 | 98.90 | 98.94 | 98.94 | 98.80±0.259 |
| Mean | 99.01±0.260 | |||||
Table 3: Gamma passing rates of EPID portal dosimetry using 3%/3mm gamma criteria
| Tumor Site | EPID portal dosimetry | |||||
|---|---|---|---|---|---|---|
| Gamma passing rates (2%/2mm) | Average±SD | |||||
| Neck | 98.52 | 98.53 | 98.53 | 98.84 | 98.72 | 98.63±0.130 |
| Lung | 98.02 | 98.23 | 98.22 | 98.23 | 98.13 | 98.17±0.082 |
| Breast | 98.34 | 98.41 | 98.38 | 98.45 | 98.42 | 98.40±0.037 |
| Esophageal | 98.10 | 98.23 | 98.20 | 98.23 | 98.13 | 98.18±0.053 |
| Prostate | 97.45 | 98.39 | 98.40 | 98.41 | 98.43 | 98.22±0.383 |
| Rectal | 97.45 | 98.42 | 98.39 | 98.41 | 98.43 | 98.22±0.385 |
| Mean | 98.30±0.165 | |||||
Table 4: Gamma passing rates of EPID portal dosimetry using 2%/2mm gamma criteria
Overall, both the ArcCHECK™ and EPID systems demonstrated gamma passing rates above 98%, which exceeds the clinically acceptable levels. The key differences observed for each criterion are as follows. For the 3%/3mm gamma criteria, ArcCHECK™ showed higher gamma passing rates than EPID in all tumor sites except for breast, and also had lower standard deviations, indicating more consistent performance. In contrast, in the breasttumor site, EPID exhibited higher gamma passing rates than ArcCHECK™, and this difference was significant for both criteria (Figure 5). For the 2%/2mm gamma criteria, ArcCHECK™ still showed higher passing rates, but the difference between the two systems was smaller. ArcCHECK™ performed better in the lung and esophageal tumor sites, while in other sites, the difference in gamma passing rates between the two systems was relatively small.
| Tumor Site | Adj. P-value (3%3mm) | Adj. P-value (2%/2mm) |
|---|---|---|
| Neck | 0.006 | 1 |
| Lung | 0 | 0 |
| Breast | 1 | 0.138 |
| Esophageal | 1 | 0 |
| Prostate | 1 | 1 |
| Rectal | 0.042 | 0 |
Table 5: Pairwise t-test results with Bonferroni correction comparing gamma passing rates between ArcCHECKTM and EPID systems across tumor sites
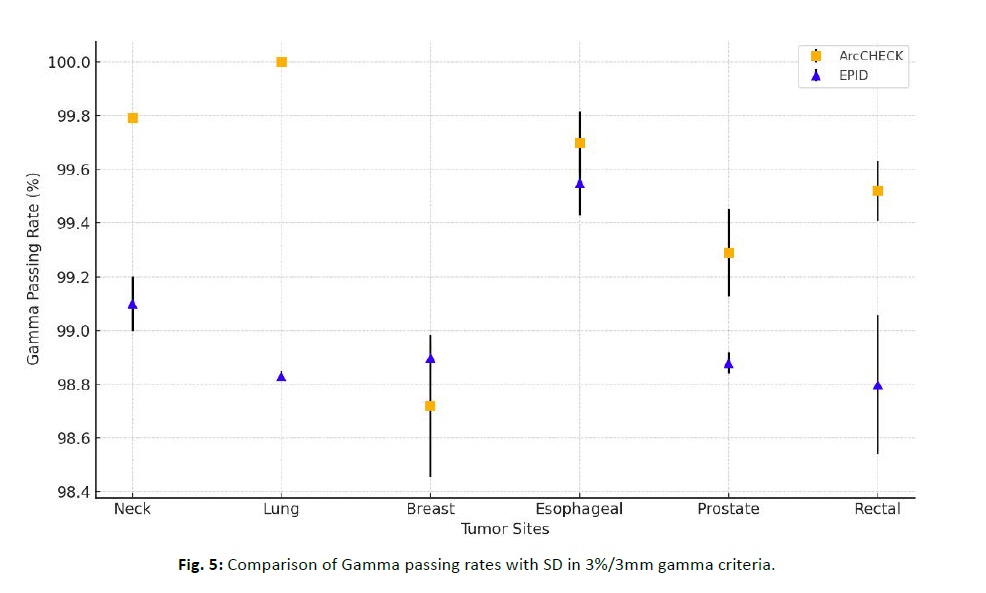
Figure 5: Comparison of Gamma passing rates with SD in 3%/3mm gamma criteria.
For the breast site, EPID showed higher gamma passing rates than ArcCHECK™, and this difference was notably significant for both criteria (Figure 6). Statistical comparisons of gamma passing rates between the ArcCHECK™ and EPID systems were conducted for each tumor site using pairwise t-tests, with Bonferroni correction applied for multiple comparisons. In the 3%/3 mm gamma criteria, statistically significant differences were observed in the neck, lung, and rectal tumor sites (p < 0.05). In contrast, there was no significant difference between the two devices in the breast, esophageal, and prostate tumor sites (p > 0.05), suggesting that both devices performed similarly in these sites. In the 2%/2 mm gamma criteria, statistically significant differences were observed in the lung and esophagealtumor sites (p < 0.05). In contrast, there was no significant difference between the two devices in the neck, breast, prostate, and rectal tumor sites (p > 0.05). Despite these statistically significant findings, all mean gamma passing rates were above 98%, indicating high dosimetric agreement and suggesting both systems are suitable for clinical DQA of VMAT plans (Table 5).
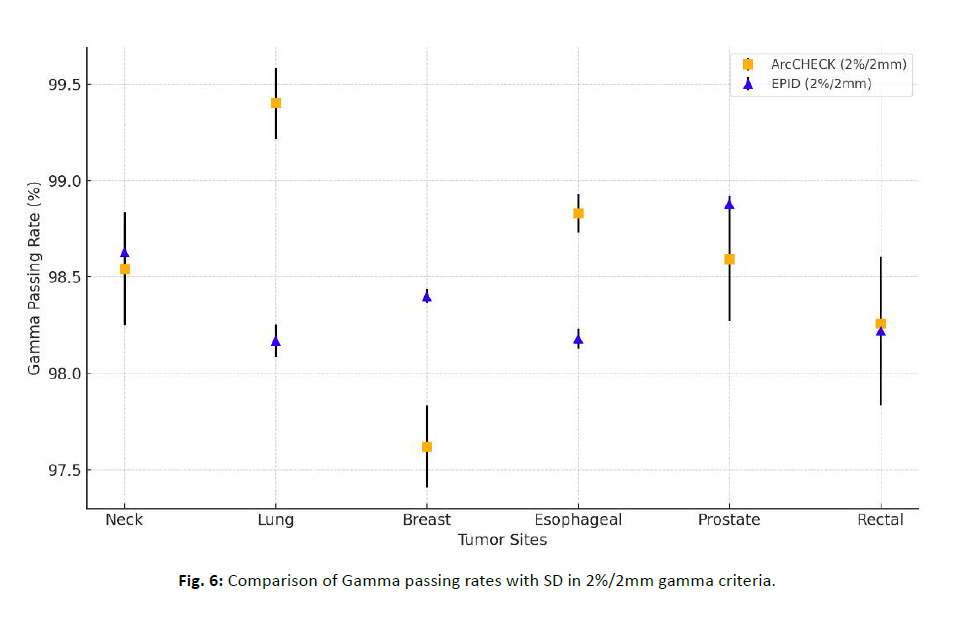
Figure 6: Comparison of Gamma passing rates with SD in 2%/2mm gamma criteria.
DISCUSSION
In this study, we compared the DQA performance of the ArcCHECK™ diode array and the EPID portal dosimetry system for VMAT treatment planning using the Halcyon™ linear accelerator. Both systems demonstrated consistently high gamma passing rates, exceeding the commonly accepted clinical threshold of 95% across all tumor sites, under both 3%/3 mm and 2%/2 mm gamma criteria.
Pairwise t-tests with Bonferroni correction revealed statistically significant differences between the two systems in certain anatomical regions. Specifically, significant differences were observed in the neck, lung, and rectal tumor sites under the 3%/3 mm gamma criteria, indicating that ArcCHECK™ showed significantly higher gamma passing rates than EPIDin these tumor sites. Significant differences were observed in the lung and esophageal tumor sites under the stricter 2%/2 mm gamma criteria, with ArcCHECK™ showing better gamma passing rates than EPID in these areas. In contrast, no significant differences were found between the two systems in the breast, prostate tumor sites under both criteria, suggesting similar performance in these tumor sites. These findings suggest that each system may exhibit different sensitivities to spatial dose variation depending on anatomical complexity and tissue heterogeneity.
The ArcCHECK™ system is particularly optimized for three-dimensional dose verification in rotational radiotherapy and has a cylindrical detector configuration that enables multidirectional dose measurement. This allows for more accurate assessment of complex dose distributions, especially in sites with significant anatomical variation such as the lung and neck. The high gamma passing rate observed in lung cases (100.00 ± 0.008%) further supports its strength in handling geometrically complex fields.
In contrast, the EPID portal dosimetry system provides high-resolution, two-dimensional imaging in real time, enabling rapid verification and online dose monitoring. It is highly suitable for workflow efficiency and detecting surface-level positional deviations. However, its two-dimensional nature and sensitivity to depth and off-axis dose gradients may account for the statistically lower passing rates in deeper or more heterogeneous anatomical sites. Previous studies by McCurdy et al. and Greer et al. have similarly reported that EPID-based dosimetry may underestimate or overestimate dose values in the presence of substantial tissue thickness or complex geometry, leading to reduced gamma passing rates in such conditions [19, 20].
Despite these statistically significant differences, the absolute values of the gamma passing rates remain well above clinical acceptability. This suggests that the differences may not translate into clinically meaningful variations in most routine treatment scenarios. Thus, both ArcCHECK™ and EPID systems are considered clinically reliable for patient-specific VMAT QA.
The selection between the two devices should therefore be guided by clinical context. ArcCHECK™ may be more appropriate for QA in complex cases where full three-dimensional evaluation is necessary, while EPID systems may be advantageous in high-throughput settings or when rapid real-time feedback is desired.
Ultimately, employing a tailored QA approach using both systems as complementary tools may improve the accuracy, safety, and efficiency of VMAT delivery, particularly when considering tumor location, patient anatomy, and institutional workflow priorities.
CONCLUSION
This study evaluated the validity and performance of the ArcCHECK™ diode array and EPID portal dosimetry systems in verifying VMAT treatment plans delivered by the Halcyon™ linear accelerator. Both systems demonstrated high gamma passing rates, confirming their suitability for patient-specific quality assurance in clinical radiation therapy. The ArcCHECK™ system showed advantages in accurately assessing three-dimensional dose distributions, particularly in rotational treatments, with superior performance in lung and head-and-neck sites. The EPID system, while limited to two-dimensional evaluation, offered efficient real-time monitoring and demonstrated comparable results, especially in breast treatments. Despite statistically significant differences in some tumor sites, all gamma passing rates remained above 98%, well within clinically acceptable thresholds. These findings indicate that both systems are reliable QA tools, and their selective use based on anatomical and clinical factors may optimize the safety and precision of VMAT delivery.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by (Jeong-Koo Kim) and (Sang-Wook Lee). The first draft of the manuscript was written by (Sang-Wook Lee) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical Approval
Because experimental work was conducted with Phantom, no approval of research ethics committees was required to accomplish the goals of this study.
Conflict of interest
The authors declare no conflict of interest in this article.
Funding
This research was supported by 2023 Hanseo University R&D program.
References
- National Cancer Center. Annual report of cancer statistics in Korea 2017. Goyang, Korea: National Cancer Center. 2018.[Google Scholar]
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, et al. External beam radiotherapy dose escalation for prostate cancer: a risk-adjusted analysis of long-term outcomes. Int J Radiat Oncol Biol Phys. 2012; 82:763-769. [Crossref], [Google Scholar] [PubMed]
- Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Image-guided radiotherapy: an overview. Clin Oncol (R Coll Radiol). 2007; 19:26-29.[Google Scholar]
- Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, et al. The management of respiratory motion in radiation oncology: report of AAPM Task Group 76. Med Phys. 2006; 33:3874-3900. [Crossref], [Google Scholar] [PubMed]
- Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008; 35:310-317. [Crossref], [Google Scholar] [PubMed]
- Teke T, Moros EG, Morin O. Beam modeling and VMAT verification for Halcyon™ linear accelerator. J Appl Clin Med Phys. 2019; 20:10-20.
- Michiels S, Poels K, Crijns W, Verellen D, Engels B, et al. Volumetric modulated arc therapy of head-and-neck cancer on a fast rotating O-ring linear accelerator: plan quality and delivery time comparison with a C-arm linac. Radiat Oncol. 2018; 13:201. [Crossref], [Google Scholar] [PubMed]
- Gao S, Xie C, Yang R, Han C, Wang J. Comparison of dosimetric characteristics of flattening filter-free and flattened beams in VMAT for lung cancer. J Appl Clin Med Phys. 2015; 16:251-260.[Google Scholar]
- Lim YK, Park JW, Lee KH, Kim JS. Clinical implementation of flattening filter-free beam for stereotactic body radiotherapy in lung cancer: dosimetric and clinical analysis. J Appl Clin Med Phys. 2014; 15:23-30.
- Gasic D, Persson GF, Thygesen P, Munck Af Rosenschöld PM, Specht L. Surface dose comparison of flattened and flattening filter-free beams using GafChromic™ film. Radiat Oncol. 2018; 13:201.
- Franck D, Fehrenbacher G, Lechner W. Surface and peripheral dose evaluation for flattening filter-free and conventional beams. Med Phys. 2015; 42:6565-6573.
- Zhen H, Nelms BE, Tome WA. Evaluation of the use of EPID images for IMRT dosimetry verification. Phys Med Biol. 2008; 53:2927-2944.
- Mans A, Wendling M, Mijnheer BJ, van Herk M. Cine EPID-based dosimetric verification of VMAT delivery: proof of concept. Med Phys. 2010; 37:2787-2795.
- McCurdy BM, Greer PB. Dosimetric properties of an amorphous-silicon EPID used in continuous acquisition mode for application to dynamic and intensity-modulated radiation therapy. Med Phys. 2003; 30:1618-1627. [Crossref], [Google Scholar] [PubMed]
- Wendling M, Zijp L, Mijnheer BJ. Accurate two-dimensional IMRT verification using a back-projection EPID dosimetry method. Med Phys. 2006; 33:259-273. [Crossref], [Google Scholar] [PubMed]
- Oliver M, Chen JZ, Das IJ. Investigation of the ArcCHECK QA device for IMRT and VMAT verification. Phys Med Biol. 2013; 58:4201-4215.[Google Scholar]
- Piermattei A, Fidanzio A, Azario L. Dosimetric characterization of a-Si EPID for 6 MV photon beams using a phantom for pre-treatment verification. J Appl Clin Med Phys. 2007; 8:61-78.[Google Scholar]
- Kruse JJ. On the sensitivity of gamma tests in IMRT QA. Med Phys. 2010; 37:2516-2524.[Google Scholar]
- Van Elmpt W, McDermott L, Nijsten S, Wendling M, Lambin P, et al. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother Oncol. 2008; 88:289-309. [Crossref], [Google Scholar] [PubMed]
- McCurdy BM, Greer PB, Shalev S. Dosimetric performance of an amorphous silicon electronic portal imaging device for verification of intensity modulated radiation therapy. Med Phys. 2009; 36:3028-3036. [Crossref], [Google Scholar] [PubMed]



